Summary
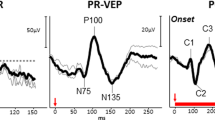
As paclitaxel may induce positive spontaneous visual symptoms or persistent visual loss, we carried out this electrophysiological study in an attempt to clarify the underlying pathophysiological mechanisms of visual pathway involvement. The study involved 30 breast cancer patients: 14 were treated with paclitaxel alone (group A) and 16 with paclitaxel and adriamycin (group B). Pattern visual evoked potentials (VEPs), and transient, 30 Hz flicker (FLK) and oscillatory potential (OP) white flash electroretinograms (ERGs), were recorded before treatment, after the third and sixth therapeutic cycle, and at the end of the programmed regimen. Pretreatment: Abnormal VEP and OP and FLK changes occurred more than 75% of patients; transient ERGs were normal in more than 90%. Serial recordings: VEPs remained unchanged in both goups. In group A, ERG b-wave latency significantly increased (ANOVA P<0.005), and OP and FLK were characterised by non-significant mild attenuation. Several combinations of ERG, OP, FLK and VEP changes occurred in 50% of the patients. The association between transitory lightining scotoma or blurred vision (reported by 12 patients) and VEP, ERG and FLK was poor, whereas that with OP was satisfactory. A few patients showed stable and persistent subclinical electrophysiological changes. Electrophysiological changes during treatment revealed the involvement of both the retina and anterior optic pathway. There was only a weak correlation between visual symptoms and electrophysiology. We suggest that the most likely mechanism of visual symptoms and electrophysiological changes during paclitaxel administration is vascular dysregulation in the retina, or ischemic mechanisms when the optic nerve is involved.
Similar content being viewed by others
References
Ekholm E, Rantanen V, Antila K, Salminen E, Paclitaxel changes sympathetic control of blood pressure Eur J Cancer 1997; 33(9):1419–1424
Ekholm E, Rantanen V, Bergman M, Vesalainen R, Antila K, Salminen E, Docetaxel and autonomic cardiovascular control in anthracycline treated breast cancer patients Anticancer Res 2000; 20(3B):2045–2048
Faivre S, Goldwasser F, Soulie P, Misset JL, Paclitaxel (Taxol)-associated junctional tachycardia Anticancer Drugs 1997; 8(7):714–716
Papadopoulos KP, Egorin MJ, Huang M, Troxel AB, Kaufman E, Balmaceda CM, et al. The pharmacokinetics and pharmacodynamics of high-dose paclitaxel monotherapy (825 mg/m2 continuous infusion over 24 h) with hematopoietic support in women with metastatic breast cancer Cancer Chemother Pharmacol 2001; 47(1):45–50
Gianni L, Dombernowsky P, Sledge G, Martin M, Amadori D, Arbuck SG, et al. Cardiac function following combination therapy with paclitaxel and doxorubicin: an analysis of 657 women with advanced breast cancer Ann Oncol 2001; 12(8):1067–1073
Hagiwara H, Sunada Y, Mechanism of taxane neurotoxicity Breast Cancer 2004; 11(1):82–85
Johnson DW, Cagnoni PJ, Schossau TM, Stemmer SM, Grayeb DE, Baron AE, et al. Optic disc and retinal microvasculopathy after high-dose chemotherapy and autologous hematopoietic progenitor cell support Bone Marrow Transplant 1999; 24(7):785–792
Scaioli V, Caraceni A, Martini C, Palazzini E, Tarenzi E, Fulfaro F, et al. Visual evoked potentials findings in course of paclitaxel doxorubicin combination chemotherapy Report of a case J Neurooncol 1995; 25(3):221–225
Tan WW, Walsh T Ocular toxicity secondary to paclitaxel in two lung cancer patients Med Pediatr Oncol 1998; 31(3):177
Teitelbaum BA, Tresley DJ, Cystic maculopathy with normal capillary permeability secondary to docetaxel Optom Vis Sci 2003; 80(4):277–279
Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC, Neurotoxicity of Taxol J Natl Cancer Inst Monogr 1993;(15):107–115
Ziske CG, Schottker B, Gorschluter M, Mey U, Kleinschmidt R, Schlegel U, et al. Acute transient encephalopathy after paclitaxel infusion: report of three cases Ann Oncol 2002; 13(4):629–631
Perry JR, Warner E, Transient encephalopathy after paclitaxel (Taxol) infusion Neurology 1996; 46(6):1596–1599
Nieto Y, Cagnoni PJ, Bearman SI, Shpall EJ, Matthes S, DeBoom T, et al. Acute encephalopathy: a new toxicity associated with high-dose paclitaxel Clin Cancer Res 1999; 5(3):501–506
McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms Ann Intern Med 1989; 111(4):273–279
Brown T, Havlin K, Weiss G, Cagnola J, Koeller J, Kuhn J, et al. A phase I trial of taxol given by a 6-hour intravenous infusion J Clin Oncol 1991; 9(7):1261–1267
Ziske CG, Schottker B, Gorschluter M, Mey U, Kleinschmidt R, Schlegel U, et al. Acute transient encephalopathy after paclitaxel infusion: report of three cases Ann Oncol 2002; 13(4):629–631
Vaphiades MS, Celesia GG, Brigell MG, Positive spontaneous visual phenomena limited to the hemianopic field in lesions of central visual pathways Neurology 1996; 47(2):408–417
Scaioli V, Antozzi C, Villani F, Rimoldi M, Zeviani M, Panzica F, et al. Utility of multimodal evoked potential study and electroencephalography in mitochondrial encephalomyopathy Ital J Neurol Sci 1998; 19(5):291–300
Kergoat H, Lovasik JV, The effects of altered retinal vascular perfusion pressure on the white flash scotopic ERG and oscillatory potentials in man Electroencephalogr Clin Neurophysiol 1990; 75(4):306–322
Kergoat H, Electroretinogram in unilateral vascular stress in nondiabetic and diabetic subjects Optom Vis Sci 1993; 70(9):743–749
Lovasik JV, Kergoat H, Influence of transiently altered retinal vascular perfusion pressure on rod/cone contributions to scotopic oscillatory potentials Ophthalmic Physiol Opt 1991; 11(4):370–380
Tinjust D, Kergoat H, Lovasik JV, Neuroretinal function during mild systemic hypoxia Aviat Space Environ Med 2002; 73(12):1189–1194
Coleman K, Fitzgerald D, Eustace P, Bouchier-Hayes D, Electroretinography, retinal ischaemia and carotid artery disease Eur J Vasc Surg 1990; 4(6):569–573
Hara A, Miura M, Decreased inner retinal activity in branch retinal vein occlusion Doc Ophthalmol 1994; 88(1):39–47
Holopigian K, Seiple W, Lorenzo M, Carr R, A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy Invest Ophthalmol Vis Sci 1992; 33(10):2773–2780
Rumi V, Angelini L, Scaioli V, D’Angelo A, Besana C, Primary antiphospholipid syndrome and neurologic events Pediatr Neurol 1993; 9(6):473–475
Kaiser-Kupfer MI, Kupfer C, Rodrigues MM, Tamoxifen retinopathy. A clinicopathologic reportOphthalmology 1981; 88(1):89–93
Toimela T, Tahti H, Salminen L, Retinal pigment epithelium cell culture as a model for evaluation of the toxicity of tamoxifen and chloroquine Ophthalmic Res 1995; 27(Suppl 1):150–153
Zhang JJ, Jacob TJ, Valverde MA, Hardy SP, Mintenig GM, Sepulveda FV, et al. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation J Clin Invest 1994; 94(4):1690–1697
Dulley P., Ocular adverse reactions to tamoxifen–a review Ophthalmic Physiol Opt 1999; 19(Suppl) 1:S2--S9
Flach AJ, Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity: a prospective study of 63 patients Surv Ophthalmol 1994; 38(4):392–393
Ah-Song R, Sasco AJ, Tamoxifen and ocular toxicity Cancer Detect Prev 1997; 21(6):522–531
De Laey JJ, Flecked retina disorders Bull Soc Belge Ophtalmol 1993; 249:11–22
Costa RH, Dhooge MR, Van Wing F, De Rouck AF, Tamoxifen retinopathy. A case report Bull Soc Belge Ophtalmol 1990; 238:161–168
Noureddin BN, Seoud M, Bashshur Z, Salem Z, Shamseddin A, Khalil A Ocular toxicity in low-dose tamoxifen: a prospective study Eye 1999; 13(Pt 6):729–733
Vinding T, Nielsen NV Retinopathy caused by treatment with tamoxifen in low dosage Acta Ophthalmol (Copenh) 1983; 61(1):45–50
Pugesgaard T, Von Eyben FE, Bilateral optic neuritis evolved during tamoxifen treatment Cancer 1986; 58(2):383–386
Ashford AR, Donev I, Tiwari RP, Garrett TJ, Reversible ocular toxicity related to tamoxifen therapy Cancer 1988; 61(1):33–35
Colley SM, Elston JS, Tamoxifen optic neuropathy Clin Experiment Ophthalmol 2004; 32(1):105–106
Ostrow S, Hahn D, Wiernik PH, Richards RD, Ophthalmologic toxicity after cis-dichlorodiammineplatinum(II) therapy Cancer Treat Rep 1978; 62(10):1591–1594
Rubin P, Hulette C, Khawly JA, Elkordy M, Hussein A, Vredenburgh JJ, et al. Ocular toxicity following high dose chemotherapy and autologous transplant Bone Marrow Transplant 1996; 18(1):253–256
Johnson DW, Cagnoni PJ, Schossau TM, Stemmer SM, Grayeb DE, Baron AE, et al. Optic disc and retinal microvasculopathy after high-dose chemotherapy and autologous hematopoietic progenitor cell support Bone Marrow Transplant 1999; 24(7):785–792
Author information
Authors and Affiliations
Corresponding author
Additional information
Address for offprints: Vidmer Scaioli, C. Besta National Insitute of Neurology, Via Celoria 11, 20133 Milano, Italy; Tel.: +39-02-2394275; Fax: +39-02-70600775; E-mail: vscaioli@istituto-besta.it
Rights and permissions
About this article
Cite this article
Scaioli, V., Caraceni, A., Martini, C. et al. Electrophysiological evaluation of visual pathways in paclitaxel-treated patients. J Neurooncol 77, 79–87 (2006). https://doi.org/10.1007/s11060-005-9008-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-005-9008-x