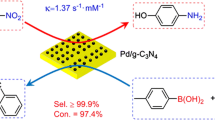
Abstract
Herein, six kinds of PdNPs (including icosahedron, sphere, spindle, cube, rod, and wire) were synthesized via simple methods. The catalytic activities were investigated by the reduction reaction of Cr(VI) and Suzuki coupling reaction. Chemically synthesized morphologies of the six catalysis were characterized by transmission electron microscopy, field emission scanning electron microscopy, and X-ray diffraction, etc. Pd icosahedron shows a better catalytic property than other PdNPs with a rate constants 0.42 min−1 for the reduction of Cr(VI). Moreover, the electrocatalyst shows that Pd icosahedron possesses a bigger surface area of 8.56 m2/g than other nanoparticles, which is attributed to the better catalyst. The Pd icosahedron possesses a better catalytic property, attributing to the abundant exposed {111} facets with high activity on Pd icosahedron. The catalytic activities are closely related to the surface area with the following order: icosahedrons ≥ sphere > rod > spindle > cube > wire. The Pd icosahedron catalyst represents a strong activity for Suzuki coupling reaction as well, outweighting is 80%. The results reveal that Pd icosahedron acts as an efficient catalyst compared to other PdNPs (wire, rod, sphere, spindle, and cube).









Similar content being viewed by others
References
Bhowmik K, Mukherjee A, Mishra MK, De G (2014) Stable Ni nanoparticle–reduced graphene oxide composites for the reduction of highly toxic aqueous Cr (VI) at room temperature. Langmuir 30:3209–3216
Borah BJ, Saikia H, Bharali P (2014) Reductive conversion of Cr (VI) to Cr (III) over bimetallic CuNi nanocrystals at room temperature. New J Chem 38:2748–2751
Brieger G, Nestrick TJ (1974) Catalytic transfer hydrogenation. Chem Rev 74:567–580
Buerge IJ, Hug SJ (1998) Environ. Sci& Technol 32:2092–2099
Calo V, Nacci A, Monopoli A, Montingelli F (2005) Pd nanoparticles as efficient catalysts for Suzuki and Stille coupling reactions of aryl halides in ionic liquids. J Org Chem 70:6040–6044
Celebi M, Yurderi M, Bulut A, Kaya M, Zahmakiran M (2016) Palladium nanoparticles supported on amine-functionalized SiO2 for the catalytic hexavalent chromium reduction. Appl Catal B Environ 180:53–64
Chen Y-H, Hung H-H, Huang MH (2009a) Seed-mediated synthesis of palladium nanorods and branched nanocrystals and their use as recyclable Suzuki coupling reaction catalysts. J Am Chem Soc 131:9114–9121
Chen Y-H, Hung H-H, Huang MH (2009b) Seed-mediated synthesis of palladium nanorods and branched nanocrystals and their use as recyclable Suzuki coupling reaction catalysts. J Am Chem Soc 131:9114–9121
Dandapat A, Jana D, De G (2011) Pd nanoparticles supported mesoporous γ-al 2 O 3 film as a reusable catalyst for reduction of toxic Cr VI to Cr III in aqueous solution. Appl Catal A 396:34–39
Fu G, Jiang X et al (2013) Polyallylamine functionalized palladium icosahedra: one-pot water-based synthesis and their superior electrocatalytic activity and ethanol tolerant ability in alkaline. Langmuir 29:4413–4420
Fu G-T, Jiang X et al (2014a) Arginine-assisted synthesis and catalytic properties of single-crystalline palladium tetrapods. ACS Appl Mater& Inter 6:22790–22795
Fu G-T, Jiang X et al (2014b) Arginine-assisted synthesis and catalytic properties of single-crystalline palladium tetrapods. ACS Appl. Mater. & Inter. 6:22790–22795
Guo Y, Wang D, Liu X, Wang X, Liu W, Qin W (2014) Synthesis and characterization of the nickel@ carbon dots hybrid material and its application in the reduction of Cr (VI). New J Chem 38:5861–5867
Han J, Liu Y, Guo R (2009) Facile synthesis of highly stable gold nanoparticles and their unexpected excellent catalytic activity for Suzuki− Miyaura cross-coupling reaction in water. J Am Chem Soc 131:2060–2061
Hsu L, Wang S, Lin Y, Wang M et al (2011) One-pot synthesis of magnetic graphene nanocomposites decorated with core@ double-shell nanoparticles for fast chromium removal. Environ Sci& Technol 44:6202–6208
Hu C, Ting S-W, Chan K-Y, Huang W (2012a) Int. reaction pathways derived from DFT for understanding catalytic decomposition of formic acid into hydrogen on noble metals. J Hydrogen Ener 37:15956–15965
Hu C, Ting S-W, Chan K-Y, Huang W (2012b) Reaction pathwaysderived from DFT for understanding catalytic decomposition offormic acid into hydrogen on Noble metals. Int. J. Hydrogen Energy 37:15956–15965
Huang X, Zheng N (2009) One-pot, high-yield synthesis of 5-fold twinned Pd nanowires and nanorods. J Am Chem Soc 131:4602–4603
Huang Y, Ma H et al (2012) Efficient catalytic reduction of hexavalent chromium using palladium nanoparticle-immobilized electrospun polymer nanofibers. ACS Appl. Mater. & Inter. 4:3054–3061
Johnstone RA, Wilby AH, Entwistle ID (1985) Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds. Chem Rev 85:129–170
Kieber RJ, Zhou X, Mopper K (1990) Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters: fate of riverine carbon in the sea. Limnol Oceanog 35:1503–1515
Kim S-W, Kim M, Lee WY, Hyeon T (2002) Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for Suzuki coupling reactions. J Am Chem Soc 124:7642–7643
Krishnani KK, Srinives S, Mohapatra B et al (2013) Hexavalent chromium removal mechanism using conducting polymers. J Hazard Mater 252:99–106
Lan Y, Deng B, Kim C, Thornton EC, Xu H (2005) Catalysis of elemental sulfur nanoparticles on chromium (VI) reduction by sulfide under anaerobic conditions. Environ. Sci. & Technol. 39:2087–2094
Li C, Sato R, Kanehara M, Zeng H, Bando Y, Teranishi T (2009) Controllable polyol synthesis of uniform palladium icosahedra: effect of twinned structure on deformation of crystalline lattices. Angew Chem 121:7015–7019
Y.Liu, C.Khemtong,J.Hu (2004). Synthesis and catalytic activity of a poly(N,N-dialkylcarbodiimide)/palladium nanoparticle composite: a case in the Suzuki coupling reaction using microwave and conventional heating. Chem. Commun.398–399.
Lv T, Wang Y et al (2013) Controlled synthesis of Nanosized palladium icosahedra and their catalytic activity towards formic-acid oxidation. ChemSusChem 6:1923–1930
Miyaura N, Yanagi T (1981) The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. A. Suzuki. Synthetic Commun 11:513–519
Mori K, Dojo M, Yamashita H (2013) Pd and Pd–ag nanoparticles within a macroreticular basic resin: an efficient catalyst for hydrogen production from formic acid decomposition. ACS Catal 6:1114–1119
Omole MA, K’Owino IO, Sadik OA (2007) Palladium nanoparticles for catalytic reduction of Cr (VI) using formic acid. Appl Catal B Environ 76:158–167
Omole MA, Okello VA, Lee V, Zhou L, Sadik OA, Umbach C, Sammakia B (2011) Catalytic reduction of hexavalent chromium using flexible nanostructured poly (amic acids). ACS Catal 1:139–146
Qian A, Liao P, Yuan S, Luo M (2014) Efficient reduction of Cr (VI) in groundwater by a hybrid electro-Pd process. Water Res 48:326–334
Rivero-Huguet M, Marshall WD (2009) Reduction of hexavalent chromium mediated by micro-and nano-sized mixed metallic particles. J Hazard Mater 169:1081–1087
Sadik OA, Noah NM, Okello VA, Sun Z (2013) Catalytic reduction of hexavalent chromium using palladium nanoparticles: an undergraduate nanotechnology Laboratory. J Chem Educ 91:269–273
Senthil Kumar SM, Soler Herrero J, Irusta S, Scott K (2010) The effect of pretreatment of Vulcan XC-72R carbon on morphology and electrochemical oxygen reduction kinetics of supported Pd nano-particle in acidic electrolyte. J Electroanal Chem 647:211–221
Shirzad-Siboni M, Farrokhi M et al (2014) Photocatalytic reduction of hexavalent chromium over ZnO nanorods immobilized on kaolin. Ind& Eng Chem Res 53:1079–1087
Thathagar MB, Beckers J, Rothenberg G (2002) Copper-catalyzed Suzuki cross-coupling using mixed nanocluster catalysts. J Am Chem Soc 124:11858–11859
Tian Y, Huang L, Zhou X, Wu C (2012) Electroreduction of hexavalent chromium using a polypyrrole-modified electrode under potentiostatic and potentiodynamic conditionsJ. Hazard Mater 225:15–20
Vospernik M, Pintar A, Levec J (2006) Application of a catalytic membrane reactor to catalytic wet air oxidation of formic acid. Chem Eng Process 45:404–414
Wang F, Li C et al (2011) Heteroepitaxial growth of high-index-faceted palladium Nanoshells and their catalytic performance. J Am Chem Soc 133:1106–1111
Wei L-L, Gu R, Lee J-M (2015) Highly efficient reduction of hexavalent chromium on amino-functionalized palladium nanowires. Appl Catal B Environ 176-177:325–330
Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: simple Chemistry meets complex physics? Angew Chem Int Edit 48:60–103
Yadav M, Xu Q (2013) Catalytic chromium reduction using formic acid and metal nanoparticles immobilized in a metal–organic framework. Chem Commun 49:3327–3329
Yuan P, Fan M et al (2009) Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr (VI)] from aqueous solutions. J Hazard Mater 166:821–829
Zhang R, Liu H, Wang B, Ling L (2012a) Insights into the preference of CO2 formation from HCOOH decomposition on Pd surface: a theoretical study. J Phys Chem C 116:22266–22280
Zhang H, Jin M, Xiong Y, Lim B, Xia Y (2012b) Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Accounts Chem Res 46:1783–1794
Zhang R, Liu H, Wang B, Ling L (2012c) Insights into thepreference of CO2formation from HCOOH decomposition on Pdsurface: atheoretical study. J PhysChem C 116:22266–22280
Zhang L, Lu D, Chen Y, Tang Y, Lu T (2014) Facile synthesis of Pd–co–P ternary alloy network nanostructures and their enhanced electrocatalytic activity towards hydrazine oxidation. J Mater Chem A 2:1252–1256
Zheng M, Li P, Fu G, Chen Y, Zhou Y, Tang Y, Lu T (2013) Efficient anchorage of highly dispersed and ultrafine palladium nanoparticles on the water-soluble phosphonate functionalized multiwall carbon nanotubes. Appl Catal B-Environ 129:394–402
Zhou WP, Lewera A (2006) Size effects in electronic and catalytic properties of unsupported palladium nanoparticles in electrooxidation of formic acid. J Phys Chem B 110:13393–13398
Zhou S, Qian C, Chen X (2011) Comparative theoretical study ofadsorption and dehydrogenation of formic acid, hydrazine andisopropanol on Pd (111) surface. Catal Lett 141:726–734
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was financially supported by the Natural Science Foundation of China (No. 21271094).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 322 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Guo, Y., Iqbal, A. et al. Palladium nanoparticles as catalysts for reduction of Cr(VI) and Suzuki coupling reaction. J Nanopart Res 19, 150 (2017). https://doi.org/10.1007/s11051-017-3829-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3829-3