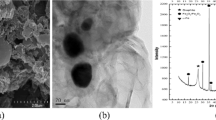
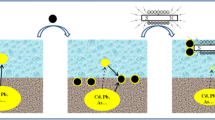
Abstract
The objective of this study was to investigate the possibility of using supported nanoscale zero-valent iron with bentonite and kaolinite for immobilization of As, Pb and Zn in contaminated sediment from the Nadela river basin (Serbia). Assessment of the sediment quality based on the pseudo-total metal content (As, Pb and Zn) according to the corresponding Serbian standards shows its severe contamination, such that it requires disposal in special reservoirs and, if possible, remediation. A microwave-assisted sequential extraction procedure was employed to assess potential metal mobility and risk to the aquatic environment. According to these results, As showed lower risk to the environment than Pb and Zn, which both represent higher risk to the environment. The contaminated sediment, irrespective of the different speciation of the treated metals, was subjected to the same treatment. Semi-dynamic leaching test, based on leachability index and effective diffusion coefficients, was conducted for As-, Pb- and Zn-contaminated sediments in order to assess the long-term leaching behaviour. In order to simulate “worst case” leaching conditions, the test was modified using acetic and humic acid solution as leachants instead of deionized water. A diffusion-based model was used to elucidate the controlling leaching mechanisms; in the majority of samples, the controlling leaching mechanism appeared to be diffusion. Three different single-step leaching tests were applied to evaluate the extraction potential of examined metals. Generally, the test results indicated that the treated sediment is safe for disposal and could even be considered for “controlled utilization”.




Similar content being viewed by others
References
Alidokht L, Khataee AR, Reyhanitabar A, Oustan S (2011) Cr(VI) immobilization process in a Cr-spiked soil by zerovalent iron nanoparticles: optimization using response surface methodology. Clean Soil Air Water 39:633–640
ANS (American National Standard) ANSI/ANS 16.1 (1986) American National Standard for the measurement of the leachability of solidified low-level radioactive wastes by short-term tests procedures. American National Standards Institute, New York
ASTM D1557-00 Standard test method for laboratory compaction characteristics of soil using modified effort American Society for Testing Materials. Annual book of ASTM standards: ASTM D1557-91, vol 4.08. ASTM, Philadelphia
Ayodele OB, Hameed BH (2013) Development of kaolinite supported ferric oxalate heterogeneous catalyst for degradation of 4-nitrophenol in photo-Fenton process. Appl Clay Sci 83–84:171–181
Barajas-Aceves M, Corona-Hernandez J, Rodrıguez-Vazquez R (2007) Chromium fractionation in semi-arid soils amended with chromium and tannery sludge. J Hazard Mater 146:91–97
Barcelo D, Petrovic M (2007) Sustainable management of sediment resources: sediment quality and impact assessment of pollutants. Elsevier, Amsterdam
Baudo R, Giesy J, Muntau H (1990) Sediments: chemistry and toxicity of in-place pollutants. CRC Press Inc., Boca Raton
Bhattacharyya KG, Gupta SS (2006) Adsorption of chromium(VI) from water by clays. Ind Eng Chem Res 45(21):7232–7240
CCR, California Code of Regulations (1998) Title 22, Chapter 11, Article 5, Appendix II
Chrysochoou M, Johnston CP, Dahal G (2012) A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J Hazard Mater 201:33–42
Cumbal L, Greenleaf J, Leun D, SenGupta AK (2003) Polymer supported inorganic nanoparticles: characterization and environmental applications. React Funct Polym 54:167–180
Dalmacija M, Prica M, Dalmacija B, Roncevic S, Klasnja M (2011) Quantifying the environmental impact of As and Cr in stabilized/solidified materials. Sci Total Environ 412–413:366–374
de Groot GJ, van der Sloot HA (1992) Determination of leaching characteristics of waste materials leading to environmental product certification. In: Gilliam TM, Wiles CC (eds) Stabilization and solidification of hazardous, radioactive, and mixed wastes, ASTMSTP 1123, vol 2. American Society for Testing Materials, Philadelphia, pp 149–170
Dermatas D, Meng X (2003) Utilisation of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng Geol 70:377–394
Dermatas D, Moon D, Menounou N, Meng X, Hires R (2004) An evaluation of arsenic release from monolithic solids using a modified semi-dynamic leaching test. J Hazard Mater 116:25–38
Dixit S, Hering JG (2003) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ Sci Technol 37:4182–4189
Du J, Lu J, Wu Q, Jing C (2012) Reduction and immobilization of chromate in chromite ore processing residue with nanoscale zero-valent iron. J Hazard Mater 215:152–158
Environment Canada, Proposed Evaluation Protocol for Cement-Based Solidified Wastes (1991) Environmental Protection Series. Report No. EPS 3/HA/9
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278
Franco DV, Da Silva LM, Jardim WF (2009) Reduction of hexavalent chromium in soil and ground water using zero-valent iron under batch and semi-batch conditions. Water Air Soil Pollut 197:49–60
García-Sanchez A, Alvarez-Ayuso E, Rodriguez-Martin F (2002) Sorption of As(V) by some oxyhydroxides and clay minerals. Application to its immobilization in two polluted mining soils. Clay Miner 37:187–194
Gil-Díaz MM, Pérez-Sanz A, Vicente MÁ, Lobo MC (2014) Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: effects on soil properties. Clean Soil Air Water 42:1–9
Grieger KD, Fjordbùge A, Hartmann NB, Eriksson E, Bjerg PL, Baun A (2011) Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: risk mitigation or trade-off? J Contam Hydrol 118:165–183
He F, Zhao D (2005) Preparation and characterization of a new class of starch stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environ Sci Technol 39:3314–3320
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41:6216–6221
Hizal J, Apak R (2006) Modeling of cadmium(II) adsorption on kaolinite-based clays in the presence and absence of humic acid. Appl Clay Sci 32:232–244
ISO 11277:2009 Soil quality—determination of particle size distribution in mineral soil material—method by sieving and sedimentation
Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, Kandhro GA, Shah AQ, Baig JA (2009) Speciation of heavy metals in untreated sewage sludge by using microwave assisted sequential extraction procedure. J Hazard Mater 163:1157–1164
Jing C, Liu S, Korfiatis G, Meng X (2006) Leaching behaviour of Cr(III) in stabilized/solidified soil. Chemosphere 64:379–385
Kanel SR, Nepal D, Manning B, Choi H (2007) Transport of surface-modified iron nanoparticle in porous media and application to arsenic(III) remediation. J Nanopart Res 9:725–735
Kerkez Dj, Tomašević D, Kozma G, Bečelić-Tomin M, Prica M, Rončević S, Kukovecz Á, Dalmacija B, Kónya Z (2014) Three different clay-supported nanoscale zero-valent iron materials for industrial azo dye degradation: a comparative study. J Taiwan Inst Chem Eng. doi:10.1016/j.jtice.2014.04.019
Klimkova S, Cernik M, Lacinova L, Filip J, Jancik D, Zboril R (2011) Zerovalent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere 82:1178–1184
Kumpiene J, Ore S, Renella G, Mench M, Lagerkvist A, Maurice C (2006) Assessment of zerovalent iron for stabilization of chromium, copper, and arsenic in soil. Environ Pollut 144:62–69
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manage 28:15–225
Li XQ, Zhang WX (2007) Sequestration of metal cations with zerovalent iron nanoparticles—a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phy Chem C 111:6939–6946
Li X, Elliott DW, Zhang WX (2006) Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State Mater Sci 31:111–122
Lien HL, Jhuo YS, Chen LH (2007) Effect of heavy metals on dechlorination of carbon tetrachloride by iron nanoparticles. Environ Eng Sci 24:21–30
Lund U, Fobian A (1991) Pollution of two soils by arsenic, chromium and copper. Geoderma 49:83–103
Ministry of Natural Resources, Mining and Spatial Planning. Regulation on limit values for pollutants in surface, ground water and sediment and deadlines for their achievement. The Official, Gazette 35/2011 (in Serbian)
Moore T, Rightmire C, Vempati R (2000) Ferrous iron treatment of soils contaminated with arsenic-containing wood-preserving solution. Soil Sediment Contam 9:375–405
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy remediation of dredged sediments. J Hazard Mater 85:145–163
Nathwani JS, Phillips CR (1980) Leachability of Ra-226 from uranium mill tailings consolidated with naturally occurring materials and/or cement: analysis based on mass transport equation. Water Air Soil Pollut 14:389–402
NEN 5754:1994 Determination of organic matter content in soil as loss-on-ignition
Official Gazette of RS, 67 (2011) and 48 (2012) Regulation on limit values of pollutants in water and deadlines for their achievement
Pagnanelli F, Moscardini E, Giuliano V, Toro L (2004) Sequential extraction of heavy metals in river sediments of an abandoned pyrite mining area: pollution detection and affinity series. Environ Pollut 132:89–201
Pawlett M, Ritz K, Dorey RA, Rocks S, Ramsden J, Harris JA (2013) The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ Sci Pollut Res 20:1041–1049
Phenrat T, Marhaba T, Rachakornkij M (2007) XRD and unconfined compressive strength study for a qualitative examination of calcium–arsenic compounds retardation of cement hydration in solidified/stabilized arsenic–iron hydroxide sludge. J Environ Eng 133:595–607
Quevauviller P (2002) Methodologies in soil and sediment fractionation studies: single and sequential extraction procedures. The Royal Society of Chemistry, London
Richmond WR, Loan M, Morton J, Parkinson GM (2004) Arsenic removal from aqueous solution via ferrihydrite crystallization control. Environ Sci Technol 38:2368–2372
Shi L, Lin Y, Zhang X, Chen Z (2011) Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution. Chem Eng J 171:612–617
Son YH, Lee JK, Soong Y, Martello D, Chyu MK (2012) Heterostructured zero valent iron–montmorillonite nanohybrid and their catalytic efficacy. Appl Clay Sci 62–63:21–26
Stumm W, Morgan J (1996) Aquatic chemistry: chemical equilibria and rates in natural waters (Chapter 6), 3rd edn. Wiley, New York
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Townsend T, Dubey B, Tolaymat T, Solo-Gabriele H (2005) Preservative leaching from weathered CCA-treated wood. J Environ Manage 75:105–113
USEPA (2002a) Synthetic precipitation leaching procedure, method 1312. Internet site: http://www.EPA.gov/SW-846/1312.pdf
USEPA (2002b) Toxicity characteristic leaching procedure, method 1311. Internet site: http://www.EPA.gov/SW-846/1311.pdf
USEPA Method 7010 (2007) Graphite furnace absorption spectrophotometry, Revision 0
USEPA Method 3051a (2007) Microwave assisted acid digestion of sediments, sludges, soils and, Revision 1
Warren GP, Alloway BJ (2003) Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil. J Environ Qual 32:767–772
Yan WL, Herzing AA, Kiely CJ, Zhang WX (2010a) Nanoscale zero-valent iron (nZVI): aspects of the core–shell structure and reactions with inorganic species in water. J Contam Hydrol 118:96–104
Yan WL, Ramos MAV, Koel BE, Zhang WX (2010b) Multi-tiered distributions of arsenic in iron nanoparticles: observation of dual redox functionality enabled by a core–shell structure. Chem Commun 46:6995–6997
Zeng L (2003) A method for preparing silica-containing iron(III) oxide adsorbents for arsenic removal. Water Res 37:4351–4358
Acknowledgments
This work has been produced with the financial assistance of the EU (Project MATCROSS, HUSRB 1002/214/188) and the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project Numbers III43005 and TR37004). The financial support of the TÁMOP-4.2.2.A-11/1/KONV-2012-0047, TÁMOP-4.2.2.A-11/1/KONV-2012-0060 and OTKA NN 110676 projects is acknowledged. The contents of this document are the sole responsibility of the University of Novi Sad Faculty of Sciences and can under no circumstances be regarded as reflecting the position of the European Union and/or the Managing Authority.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomašević, D.D., Kozma, G., Kerkez, D.V. et al. Toxic metal immobilization in contaminated sediment using bentonite- and kaolinite-supported nano zero-valent iron. J Nanopart Res 16, 2548 (2014). https://doi.org/10.1007/s11051-014-2548-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2548-2