Abstract
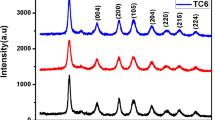
Hydrothermal preparation of pure anatase TiO2 with hybrid nano and micro-morphologies directly from titania sol under acidic condition in the absence of any additives or templates has rarely been reported. The present work has found that the post-hydrothermal treatment at 200 °C for different times (6, 12, 24, and 36 h) of titania sol under an acidic environment affected strongly on the structural, morphology, and optical properties of TiO2. A single-crystalline anatase phase with high surface area was obtained. The TEM results showed that shape of TiO2 nanoparticles could be manipulated by post-hydrothermal treatment. The increasing of hydrothermal time (pH 2.5) significantly altered the morphology of TiO2 from pure aggregated nanospherical shape (6 h) into branched micro-flowers as a major shape in addition to nanorod, nanocube, and nanosphere shapes (24 h). Shape-controlled TiO2 nanoparticles showed a red shift in UV–Vis light reflectance spectra as compared to TiO2 nanoparticles obtained without any hydrothermal treatment. The photoluminescence measurements confirm that hydrothermal treatment significantly decrease the electron–hole recombination chance in the obtained TiO2. The fluorescent probe method was used for evaluation of the photo-oxidative activity of different TiO2 nanomaterials. The highly active TiO2 nanoparticle (hydrothermally treated for 24 h) was applied for industrial wastewater treatment using solar radiation as a renewable energy source.







Similar content being viewed by others
References
Andersson M, Osterlund L, Ljungstrom S, Palmqvist A (2002) Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J Phys Chem B 106:10674–10679
Asal S, Saif M, Hafez H, Mozia S, Heciak A, Moszyński D, Abdel-Mottaleb MSA (2011) Photocatalytic generation of useful hydrocarbons and hydrogen from acetic acid in the presence of lanthanide modified TiO2. Int J Hydrogen Energy 36:6529–6537
Bischof BL, Anderson MA (1995) Peptization process in the sol gel preparation of porous anatase (TiO2). Chem Mater 7:1772–1778
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) The chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Byrappa K, Yoshimura M (2001) Handbook of hydrothermal technology. William Andrew Publishing, New York
Carotta MC, Gherardi S, Malagù C, Nagliati M, Vendemiati B, Martinelli G, Sacerdoti M, Lesci IG (2007) Comparison between titania thick films obtained through sol–gel and hydrothermal synthetic processes. Thin Solid Films 515:8339–8344
Chena L-C, Huang C-M, Tsai F-R (2007) Characterization and photocatalytic activity of K+-doped TiO2 photocatalysts. J Mol Catal A Chem 265:133–140
Cheng H, Ma J, Zhao Z, Qi L (1995) Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem Mater 7:663–671
D’Elia D, Beauger C, Hochepied J-F, Rigacci A, Berger M-H, Keller N, Keller-Spitzer V, Suzuki Y, Valmalette J-C, Benabdesselam M, Achard P (2011) Impact of three different TiO2 morphologies on hydrogen evolution by methanol assisted water splitting: nanoparticles, nanotubes and aerogels. Inter J Hydrogen Energy 36:14360–14373
Eiden-Assmann S, Widoniak J, Maret G (2004) Synthesis and characterization of porous and nonporous monodisperse colloidal TiO2 particles. Chem Mater 16:6–11
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341
Fuerte A, Hernández-Alonso MD, Maira AJ, Martinez-Arias A, Fernández-Garcia M, Conesa JC, Soria J, Munuera G (2002) Nanosize Ti–W mixed oxides: effect of doping level in the photocatalytic degradation of toluene using sunlight-type excitation. J Catal 212:1–9
Guan H, Zhu L, Zhou H, Tang H (2008) Rapid probing of photocatalytic activity on titania-based self-cleaning materials using 7-hydroxycoumarin fluorescent probe. Analytica chim acta 608:73–78
Hafez H, Saif M, Mcleskey JT Jr, Abdel-Mottaleb MSA, Yahia IS, Story T, Knoff W (2009) Hydrothermal preparation of Gd3+-doped titanate nanotubes: magnetic properties and photovoltaic performance. Int J Photoenergy 2009:1–8. doi:10.1155/2009/240402
Hafez H, Saif M, Abdel-Mottaleb MSA (2011) Down-converting lanthanide doped TiO2 photoelectrodes for efficiency enhancement of dye-sensitized solar cells. J Power Sources 196:5792–5796
Han T-Y, Wu C-F, Hsieh C-T (2007) Hydrothermal synthesis and visible light photocatalysis of metal-doped titania nanoparticles. J Vac Sci Technol B 25:430–435
Ishibashi K-i, Fujishima A, Watanabe T, Hashimoto K (2000) Quantum yields of active oxidative species formed on TiO2 photocatalyst. J Photochem Photobiol A Chem 134:139–142
Jaleha B, Shayegani Madada M, Farshchi Tabrizib M, Habibia S, Golbedaghic R, Keymaneshd MR (2011) UV-degradation effect on optical and surface properties of polystyrene-TiO2 nanocomposite film. J Iran Chem Soc 8:S161–S168
Kim EJ, Hahn SH (2001) Microstructural changes of microemulsion-mediated TiO2 particles during calcination. Mater Lett 49:244–249
Kolen’ko YV, Churagulov BR, Kunst M, Mazerolles L, Colbeau-Justin C (2004a) Photocatalytic properties of titania powders prepared by hydrothermal method. Appl Catal B Environ 54:51–58
Kolen’ko YV, Maximov VD, Garshev AV, Meskin PE, Oleyni-kov NN, Churagulov BR (2004b) Hydrothermal synthesis of nanocrystalline and mesoporous titania from aqueous complex titanyl oxalate acid solutions. Chem Phys Lett 388:411–415
Kolen’ko YV, Kovnir KA, Gavrilov AI, Garshev AV, Frantti J, Lebedev OI, Churagulov BR, Tendeloo GV, Yoshimura M (2006) Hydrothermal synthesis and characterization of nanorods of various titanates and titanium dioxide. J Phys Chem B 110:4030–4038
Kumar S, Nigam N, Ghosh T, Dutta PK, Singh SP, Datta PK, An L, Shi TF (2010) Preparation, characterization and optical properties of a novel azo-based chitosan biopolymer. J Mater Chem Phys 120:361–370
Li Z, Hou B, Xu Y, Wu D, Sun Y, Hu W, Deng F (2005) Comparative study of sol–gel-hydrothermal and sol–gel synthesis of titania–silica composite nanoparticles. J Solid State Chem 178:1395–1405
Li S, Li Y, Wang H, Fan W, Zhang Q (2009) Peptization–hydrothermal method as a surfactant-free process toward nanorod-like anatase TiO2 nanocrystals. Eur J Inorg Chem 2009:4078–4084
Liao DL, Liao BQ (2007) Shape, size and photocatalytic activity control of TiO2 nanoparticles with surfactants. J Photochem Photobiol A Chem 187:363–369
Liu Z, Jin Z, Liu X, Fu Y, Liu G (2006) Fabrication of ordered TiO2 porous thin films by sol-dipping PS template method. J Sol-Gel Sci Technol 38:73–78
Lu C-H, Wen M-C (2008) Synthesis of nanosized TiO2 powders via a hydrothermal microemulsion process. J Alloys Compd 448:153–158
Monticone S, Tufeu R, Kanaev AV, Scolan E, Sanchez C (2000) Quantum size effect in TiO2 nanoparticles: does it exist? Appl Surf Sci 162–163:565–570
Morgan DL, Triani G, Blackford MG, Raftery NA, Frost RL, Waclawik ER (2011) Alkaline hydrothermal kinetics in titanate nanostructure formation. J Mater Sci 46:548–557
Nagliati M, Carotta MC, Gherardi S, Lesci IG, Martinelli G (2006) TiO2 nanopowders for sensing applications; a comparison between traditional and hydrothermal synthesis way. Adv Sci Technol 45:205–208
Neamtu M, Yediler A, Siminiceanu I, Macoveanu M, Kettrup A (2004) Decolorization of disperse red 354 azo dye in water by several oxidation processes—a comparative study. Dyes Pigments 60:61–68
Ng JD, Lorber B, Witz J, Theobald-Dietrich A, Kern D, Giege R (1996) The crystallization of biological macromolecules from precipitates: evidence for Ostwald ripening. J Cryst Growth 168:50–62
Nian J-N, Teng H (2006) Hydrothermal synthesis of single-crystalline anatase TiO2 nanorods with nanotubes as the precursor. J Phys Chem B 110:4193–4198
Penn RL (2004) Kinetics of oriented aggregation. J Phys Chem B 108:12707–12712
Piticescu RM, Piticescu RR, Taloi D, Badilita V (2003) Hydrothermal synthesis of ceramic nanomaterials for functional applications. Nanotechnology 14:312–317
Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JH, Pernicone N, Ramsay JDF, Sing KSW, Unger KK (1994) Recommendations for the characterization of porous solids. Pure Appl Chem 66:1739–1758
Roy S, Ghose J (2000) Synthesis of stable nanocrystalline cubic zirconia. Mater Res Bull 35:1195–1203
Saif M, Abdel-Mottaleb MSA (2007) Titanium dioxide nanomaterial doped with trivalent lanthanide ions of Tb, Eu and Sm: preparation, characterization and potential applications. Inorg Chim Acta 360:2863–2874
Saif M, Mashaly MM, Eid MF, Fouad R (2012) Synthesis, characterization and thermal studies of binary and/or mixed ligand complexes of Cd(II), Cu(II), Ni(II) and Co(III) based on 2-(Hydroxybenzylidene) thiosemicarbazone: DNA binding affinity of binary Cu(II) complex. Spectrochim Acta A 92:347–356
Salvador P, Gutierrez C (1982) The role of surface state in the electroreduction of dissolved and/or photogenerated oxygen on n-TiO2 electrodes. Chem Phys Lett 86:131–134
Seery MK, George R, Floris P, Pillai SC (2007) Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J Photochem Photobiol A Chem 189:258–263
Shankar MV, Anandan S, Venkatachalam N, Arabindoo B, Murugesan V (2004) Novel thin-film reactor for photocatalytic degradation of pesticides in aqueous solutions. J Chem Technol Biotechnol 79:1258–1279
Sreethawong T, Suzuki Y, Yoshikawa S (2005) Photocatalytic evolution of hydrogen over mesoporous TiO2 supported NiO photocatalyst prepared by single-step sol–gel process with surfactant template. Int J Hydrogen Energy 30:1053–1062
Tang J, Mei S, Ferreira MF (2000) Hydrothermal synthesis of nano-TiO2 powders: influence of peptisation and peptising agents on the crystalline phases and phase transitions. J Am Ceram Soc 83:1361–1368
Tayade RJ, Kulkarni RG, Jasra RV (2006) Photocatalytic degradation of aqueous nitrobenzene by nanocrystalline TiO2. Ind Eng Chem Res 45:922–927
Xiao Q, Si Z, Zhang J, Xiao C, Tan X (2008) Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J Hazard Mater 150:62–67
Xu JX, Li LP, Yan YJ, Wang H, Wang XX, Fu XZ, Li GS (2008) Synthesis and photoluminescence of well-dispersible anatase TiO2 nanoparticles. J Colloid Interface Sci 318:29–34
Yang Y, Ma J, Qin Q, Zhai X (2007) Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J Mol Catal A Chem 267:41–48
Yu Y, Xu D (2007) Single-crystalline TiO2 nanorods: highly active and easily recycled photocatalysts. Appl Catal B 73:166–171
Yu JC, Yu JG, Ho WK, Jiang ZT, Zhang LZ (2002) Effects of F− doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem Mater 14:3808–3816
Yu H, Yu J, Cheng B, Zhou M (2006a) Effects of hydrothermal post-treatment on microstructures and morphology of titanate nanoribbons. J Solid State Chem 179:349–354
Yu J, Yu H, Cheng B, Zhao X, Zhang Q (2006b) Preparation and photocatalytic activity of mesoporous anatase TiO2 nanofibers by a hydrothermal method. J Photochem Photobiol A Chem 182:121–127
Yu J, Wang G, Cheng B, Zhou M (2007) Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders. Appl Catal B Environ 69:171–180
Zhang QH, Gao L, Guo JK (2000) Effect of hydrolysis conditions on morphology and crystallisation of nanosized TiO2 powder. J Eur Ceram Soc 20:2153–2158
Zhang Y, Lan D, Wang Y, Wang F (2008) Hydrothermal synthesis of 2D ordered macroporous ZnO films. Front Chem China 3:229–234
Zhang W, Yang B, Chen J (2012) Effects of calcination temperature on preparation of boron-doped TiO2 by sol-Gel method. Int J Photoenergy Article ID: 528637
Zhong Z, Ang T-P, Luo J, Gan H-C, Gedanken A (2005) Synthesis of one-dimensional and porous TiO2 nanostructures by controlled hydrolysis of titanium alkoxide via coupling with an esterification reaction. Chem Mater 17:6814–6818
Zhu H, Gao X, Lan Y, Song D, Xi Y, Zhao J (2004) Hydrogen titanate nanofibers covered with anatase nanocrystals: a delicate structure achieved by the wet chemistry reaction of the titanate nanofibers. J Am Chem Soc 126:8380–8381
Zhu H, Lan Y, Gao X, Ringer S, Zheng Z, Song D, Zhao J (2005) Phase transition between nanostructures of titanate and titanium dioxides via simple wet-chemical reactions. J Am Chem Soc 127:6730–6736
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue Editors: Mamadou Diallo, Neil Fromer, Myung S. Jhon
This article is part of the Topical Collection on Nanotechnology for Sustainable Development
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saif, M., Aboul-Fotouh, S.M.K., El-Molla, S.A. et al. Improvement of the structural, morphology, and optical properties of TiO2 for solar treatment of industrial wastewater. J Nanopart Res 14, 1227 (2012). https://doi.org/10.1007/s11051-012-1227-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1227-4