Abstract
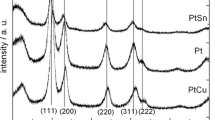
In this study, platinum nanoparticle catalysts have been prepared using PtCl4 as a starting material and 1-octanethiol, 1-decanethiol, 1-dodecanethiol, and 1-hexadecanethiol as surfactants for methanol, ethanol, and 2-propanol oxidation reactions. The structure, particle sizes, and surface morphologies of the catalysts were characterized by X-ray diffraction (XRD), atomic force microscopy and transmission electron microscopy (TEM). XRD and TEM results indicate that all prepared catalysts have a face-centered cubic structure and are homogeneously dispersed on the carbon support with a narrow size distribution (2.0–1.3 nm). X-ray photoelectron spectra of the catalysts were examined and it is found that platinum has two different oxidation states, Pt (0) and Pt(IV), oxygen and sulfur compounds are H2Oads and OHads, bound and unbound thiols. The electrochemical and electrocatalytic properties of these catalysts were investigated with respect to C1–C3 alcohol oxidations by cyclic voltammetry and chronoamperometry. The highest electrocatalytic activity was obtained from catalyst I which was prepared with 1-octanethiol. This may be attributed to a decrease in the ratio of bound to unbound thiol species increase in Pt (0)/Pt(IV), H2Oads/OHads ratios, electrochemical surface area, CO tolerance and percent platinum utility.








Similar content being viewed by others
References
Aric′o AS, Srinivasan S, Antonucci V (2001) DMFCs: from fundamental aspects to technology development. Fuel Cells 1(2):133–161. doi:10.1002/1615-6854(200107)1:2<133:AID-FUCE133>3.0.CO;2-5
Basnayake R, Li Z, Katar S, Zhou W, Rivera H, Smotkin ES, Casadonte DJ, Korzeniewski C (2006) ptru nanoparticle electrocatalyst with bulk alloy properties prepared through a sonochemical method. Langmuir 22:10446–10450. doi:10.1021/la061274o
Cao D, Bergens SH (2003) A direct 2-propanol polymer electrolyte fuel cell. J Pow Sour 124:12–17. doi:10.1016/S0378-7753(03)00613-X
Castner DG, Hinds K, Grainger DW (1996) X-ray photoelectron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces. Langmuir 12:5083–5086. doi:10.1021/la960465w
Cheng TT, Gyenge EL (2008) Efficient anodes for direct methanol and formic acid fuel cells: the synergy between catalyst and three-dimensional support. J Electrochem Soc 155(8):B819–B828. doi:10.1149/1.2932157
Colmati F, Antolini E, Gonzalez ER (2006) Effect of temperature on the mechanism of ethanol oxidation on carbon supported Pt, PtRu and Pt3Sn electrocatalysts. J Pow Sour 157:98–103. doi:10.1016/j.jpowsour.2005.07.087
Datta J, Singh S, Das S, Bandyopadhyay NR (2009) A comprehensive study on the effect of Ru addition to Pt electrodes for direct ethanol fuel cell. Bull Mater Sci 32(6):643–652
Dubau L, Hahn F, Coutanceau C, Le’ger JM, Lamy C (2003) On the structure effects of bimetallic PtRu electrocatalysts towards methanol oxidation. J Electroanal Chem 554–555:407–415. doi:10.1016/S0022-0728(03)00308-5
Eklund SE, Cliffel DE (2004) Synthesis and catalytic properties of soluble platinum nanoparticles protected by a thiol monolayer Langmuir 20(14):6012–6018. doi:10.1021/la049787n
Gardner JR, Wood R (1977) The hydrophilic nature of gold and platinum. J Electroanal Chem 81(2):285–290. doi:10.1016/S0022-0728(77)80024-7
Gökağaç G, Kennedy BJ, Cashion JD, Brown LJ (1993) Characterisation of carbon-supported Pt–Sn bimetallic catalysts for the electrochemical oxidation of methanol. J Chem Soc Farad Trans 89:151–157. doi:10.1039/FT9938900151
Goodenough JB, Hamnett A, Kennedy BJ, Manoharan R, Weeks SA (1988) Methanol oxidation on unsupported and carbon supported Pt + Ru anodes. J Electroanal Chem 240:133–145. doi:10.1016/0022-0728(88)80318-8
Gupta SS, Datta J (2005) An investigation into the electro-oxidation of ethanol and 2-propanol for application in direct alcohol fuel cells (DAFCs). J Chem Sci 117(4):337–344
Iwasita T, Hoster H, John-Anacker A, Lin WF, Vielstich W (2000) Methanol oxidation on PtRu electrodes. Influence of surface structure and Pt − Ru atom distribution. Langmuir 16:522–529. doi:10.1021/la990594n
Kabbabi A, Faure R, Durand R, Beden B, Hahn F, Leger JM, Lamy C (1998) In situ FTIRS study of the electrocatalytic oxidation of carbon monoxide and methanol at platinum–ruthenium bulk alloy electrodes. J Electroanal Chem 444:41–53. doi:10.1016/S0022-0728(97)00558-5
Kadirgan F, Beyhan S, Atilan T (2009) Preparation and characterization of nano-sized Pt–Pd/C catalysts and comparison of their electro-activity toward methanol and ethanol oxidation. Int J Hydr Energy 34(10):4312–4320. doi:10.1016/j.ijhydene.2009.03.024
Kawasaki H, Uota M, Yoshimura T, Fujikawa D, Sakai G, Kijima T (2006) One-dimensional assemblies of platinum nanoparticles on a graphite surface using nonionic/ionized mixed hemicylindrical micelle templates. J Colloid Interface Sci 300:149–154. doi:10.1016/j.jcis.2006.03.040
Kim YT, Mitani T (2006) Surface thiolation of carbon nanotubes as supports: a promising route for the high dispersion of Pt nanoparticles for electrocatalysts. J Catal 238:394–401. doi:10.1016/j.jcat.2005.12.020
Ki-Sub K, Demberelnyamba D, Lee H (2004) Size-selective synthesis of gold and platinum nanoparticles using novel thiol-functionalized ionic liquids. Langmuir 20:556–560
Klug H, Alexander L (1954) X-ray diffraction procedures, 1st edn. Wiley, New York
Liang L, Sun G, Sun S, Liu J, Tang S, Li H, Zhou B, Xin Q (2005) Structure and chemical composition of supported Pt–Sn electrocatalysts forethanol oxidation. Electrochim Acta 50:5384–5389. doi:10.1016/j.electacta.2005.03.018
Liu Z, Yu C, Russakova IA, Huang D, Strasser P (2008) Synthesis of Pt3Co alloy nanocatalyst via reverse micelle for oxygen reduction reaction in PEMFCs. Top Catal 49:241–250. doi:10.1007/s11244-008-9083-2
Mizukoshi Y, Takagi E, Okuno H, Oshima R, Maeda Y, Nagata Y (2001) Preparation of platinum nanoparticles by sonochemical reduction of the Pt(IV) ions: role of surfactants. Ultrason Sonochem 8:1–6. doi:10.1016/S1350-4177(00)00027-4
Otomo J, Li X, Kobayashi T, Wen C-J, Nagamoto H, Takahashi H (2004) AC-impedance spectroscopy of anodic reactions with adsorbed intermediates: electro-oxidations of 2-propanol and methanol on carbon-supported Pt catalyst. J Electroanal Chem 573:99–109. doi:10.1016/j.jelechem.2004.07.002
Perez H, Pradeau JP, Albouy PA, Perez O (1999) Synthesis and characterization of functionalized platinum nanoparticles. J Chem Mater 11(12):3460–3463. doi:10.1021/cm991013i
Peuckert M (1984) XPS investigation of surface oxidation layers on a platinum electrode in alkaline solution. Electrochim Acta 29(10):1315–1320. doi:10.1016/0013-4686(84)87003-6
Peuckert M, Bonzel HP (1984) Characterization of oxidized platinum surfaces by X-ray photoelectron spectroscopy. Surf Sci 145(1):239–259. doi:10.1016/0039-6028(84)90778-7
Prabhuram J, Wang X, Hui CL, Hsing IM (2003) Synthesis and characterization of surfactant-stabilized Pt/C nanocatalysts for fuel cell applications. J Phys Chem B 107:11057–11064. doi:10.1021/jp0357929
Qi Z, Hollett M, Attia A, Kaufman A (2002) Low temperature direct 2-propanol fuel cells. Electrochem Solid State Lett 5:A129–A130. http://dx.doi.org/10.1149/1.1475197
Raşa M, Kuipers BWM, Philipse AP (2002) Atomic force microscopy and magnetic force microscopy study of model colloids. J Colloid Interface Sci 250:303–315. doi:10.1006/jcis.2002.8345
Ren X, Zelenay P, Thomas S, Davey J, Gottesfeld S (2000) Recent advances in direct methanol fuel cells at Los Alamos national laboratory. J Pow Sour 86:111–116. doi:10.1016/S0378-7753(99)00407-3
Rousseau S, Coutanceau C, Lamy C, Leger JM (2006) Direct ethanol fuel cell (DEFC): electrical performances and reaction products distribution under operating conditions with different platinum-based anodes. J Pow Sour 158:18–24. doi:10.1016/j.jpowsour.2005.08.027
Schmid G (1992) Large clusters and colloids. Metals in the embryonic state. Chem Rev 92(8):1709–1727. doi:10.1021/cr00016a002
Sen F, Gokagac G (2007) Different sized platinum nanoparticles supported on carbon: an XPS study on these methanol oxidation catalysts. J Phys Chem C 111(15):5715–5720. doi:10.1021/jp068381b
Şen F, Gökağaç G (2007) Activity of carbon-supported platinum nanoparticles toward methanol oxidation reaction: role of metal precursor and a new surfactant, tert-octanethiol. J Phys Chem C 111:1467–1473. doi:10.1021/jp065809y
Sen F, Sen S, Gokagac G (2011) Efficiency enhancement of methanol/ethanol oxidation reactions on Pt nanoparticles prepared using new surfactant, 1,1-dimethyl heptanethiol. Phys Chem Chem Phys 13:1676–1684. doi:10.1039/c0cp01212b
Sumodjo TA, Silva EJ, Rabochai T (1989) Electrosorption of hydroxylated compounds: a comparative study of molecules with three carbon atoms. J Electroanal Chem 271:305–317. doi:10.1016/0022-0728(89)80084-1
Tu W, Takai K, Fuui KI, Miyazaki A, Enoki T (2003) Interface effect on the electronic structure of alkanethiol-coated platinum nanoparticles. J Phys Chem B 107(37):10134–10140. doi:10.1021/jp034738p
Wang ZB, Yin GP, Shi PF (2005) Stable Pt–Ru/C catalysts prepared from new precursors by thermal reduction for direct methanol fuel cell. J Electrochem Soc 152:A2406–12. http://dx.doi.org/10.1149/1.2120287
Watanabe M, Uchida M, Motoo S (1987) Preparation of highly dispersed Pt + Ru alloy clusters and the activity for the electrooxidation of methanol. J Electroanal Chem 229:395–406. doi:10.1016/0022-0728(87)85156-2
Woods RJ (1976) In: Bard AJ (ed) Electroanalytical chemistry, vol 9. Marcel Dekker, New York, pp 1–162
Yonezawa T, Toshima N, Wakai C, Nakahara M, Nishinaka M, Tominaga T, Nomura H (2000) Structure of monoalkyl-monocationic surfactants on the microscopic three-dimensional platinum surface in water. Colloid Surf A 169:35–45. doi:10.1016/S0927-7757(00)00414-3
Zhou WJ, Song SQ, Li WZ, Zhou ZH, Sun GQ, Xin Q, Douvartzides S, Tsiakaras P (2005) Direct ethanol fuel cells based on PtSn anodes: the effect of Sn content on the fuel cell performance. J Pow Sour 140:50–58. doi:10.1016/j.jpowsour.2004.08.003
Zubraegel Ch, Deuper C, Schneider F, Neumann M, Grunze M, Schertel A, Wöll C (1995) The presence of two different sulfur species in self-assembled films of n-alkanethiols on Au and Ag surfaces. Chem Phys Lett 238(4–6):308–312. doi:10.1016/0009-2614(95)00392-H
Acknowledgments
The authors gratefully acknowledge TÜBİTAK (Türkiye Bilimsel ve Teknik Araştırma Kurumu, Grant 108T065) for financial support and the Central Laboratory of the Middle East Technical University for acquiring XPS, TEM, and elemental analyses. The authors also thank Dr. Michael W. Pitcher for proofreading of this manuscript. F. Ş. and S. Ş thank the Middle East Technical University for Grant BAP-08-11-DPT2002K120510 and TÜBİTAK for 2211 scholarships.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Salih Ertan and Fatih Şen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ertan, S., Şen, F., Şen, S. et al. Platinum nanocatalysts prepared with different surfactants for C1–C3 alcohol oxidations and their surface morphologies by AFM. J Nanopart Res 14, 922 (2012). https://doi.org/10.1007/s11051-012-0922-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0922-5