Abstract
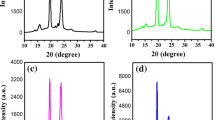
Improvement of electrical conductivity of poly ethylene oxide (PEO)–LiI polymer electrolytes is necessary for their use in solid state lithium ion battery. In this study a new kind of PEO–LiI-based polymer electrolytes embedded with CdO nanoparticles with improved electrical conductivity has been prepared and characterized. The electron microscopic studies confirm that CdO nanoparticles of average size 2.5 nm are dispersed in the PEO matrix. The glass transition temperature of the PEO–LiI electrolyte decreases with the introduction of CdO nanoparticle in the polymer matrix. X-ray diffraction, electron microscopic, and differential scanning calorimetry studies show that the amorphous phase of PEO increases with the introduction of CdO nanoparticle and that the increase in amorphous phase is maximum for 0.10 wt% CdO doping. The electrical conductivity of the sample with 0.10 wt% CdO increases by three orders in magnitude than that of the PEO–LiI electrolyte. The electrical conductivity of PEO–LiI electrolyte embedded with CdO nanoparticle exhibits VTF behavior with reciprocal temperature indicating a strong coupling between the ionic and the polymer chain segmental motions.






Similar content being viewed by others
References
Angell CA (1983) Fast ion motion in glassy and amorphous materials. Solid State Ionics 9–10:3–16
Bhattacharya S, Ghosh A (2008) Effect of ZnO nanoparticles on the structure and ionic relaxation of poly(ethylene oxide)–LiI polymer electrolyte nanocomposites. J Nanosci Nanotech 8:1922–1926
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Croce F, Curini R, Martinelli A, Persi L, Ronci F, Scrosati B (1999) Physical and chemical properties of nanocomposite polymer electrolytes. J Phys Chem B 103:10632–10638
Croce F, Persi L, Scrosati B, Fiory FS, Plichta E, Hendrickson MA (2001) Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrchimica Acta 46:2457–2461
Dias FB, Plomp L, Veldhuis JBJ (2000) Trends in polymer electrolytes for secondary lithium batteries. J Power Sources 88:169–191
Fan L, Dang Z, Wei G, Nan CW, Li M (2003) Effect of nanosized ZnO on the electrical properties of (PEO)16LiClO4 electrolytes. Mater Sci Eng B 99:340–343
Fragiadakis D, Dou S, Colby RH, Runt J (2008) Molecular mobility, ion mobility, and mobile ion concentration in poly(ethylene oxide)-based polyurethane ionomers. Macromolecules 41:5723–5728
Frech R, Chintapalli S (1996) Effect of propylene carbonate as a plasticizer in high molecular weight PEO-LiCF3SO3 electrolytes. Solid State Ionics 85:61–66
Fulcher GS (1925) Analysis of recent measurements of the viscosity of glasses. J Am Ceram Soc 8:339–355
Gadjourova Z, Andreev YG, Tunstall DP, Bruce PG (2001) Ionic conductivity in crystalline polymer electrolytes. Nature 412:520–523
Itho T, Miyamura Y, Ichikawa Y, Uno T, Kubo M, Yamamoto O (2003) Composite polymer electrolytes of poly(ethylene oxide)/BaTiO3/Li salt with hyperbranched polymer. J Power Sources 119–121:403–408
Jeong SS, Lim YT, Choi YJ, Cho GB, Kim KW, Ahn HJ, Cho KK (2007) Electrochemical properties of lithium sulfur cells using PEO polymer electrolytes prepared under three different mixing conditions. J Power Sources 174:745–750
Kim YT, Smotkin ES (2002) The effect of plasticizers on transport and electrochemical properties of PEO-based electrolytes for lithium rechargeable batteries. Solid State Ionics 149:29–37
Kumar B, Scanlon LG, Spry RJ (2001) On the origin of conductivity enhancement in polymer–ceramic composite electrolytes. J Power Sources 96:337–342
Li X, Hsu SL (1984) An analysis of the crystallization behavior of poly(ethylene oxide)/poly(methyl methacrylate) blends by spectroscopic and calorimetric techniques. J Polym Sci Polym Phys Ed 22:1331–1342
Mao G, Perea RF, Howells WS, Price DL, Saboungi ML (2000) Relaxation in polymer electrolytes on the nanosecond timescale. Nature 405:163–165
Meyer WH (1998) Polymer electrolytes for lithium-ion batteries. Adv Mater 10:439–448
Nan CW, Fan L, Lin Y, Cai Q (2003) Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys Rev Lett 91:266104
Natesan B, Karan NK, Katiyar RS (2006) Ion relaxation dynamics and nearly constant loss behavior in polymer electrolyte. Phys Rev E 74:042801
Ramesh S, Yahaya AH, Arof AK (2002) Dielectric behaviour of PVC-based polymer electrolytes. Solid State Ionics 152–153:291–294
Ribeiro R, Silva GG, Mohallen NDS (2001) A comparison of ionic conductivity, thermal behaviour and morphology in two polyether–LiI–LiAl5O8 composite polymer electrolytes. Electrchimica Acta 46:1679–1686
Scrosati B, Croce F, Persi L (2000) Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J Electrochem Soc 147:1718–1721
Scrosati B, Croce F, Panero S (2001) Progress in lithium polymer battery R&D. J Power Sources 100:93–100
Soderlund J, Kiss LB, Niklasson GA, Granqvist CG (1998) Lognormal size distributions in particle growth processes without coagulation. Phys Rev Lett 80:2386–2388
Stoeva Z, Litas IM, Staunton E, Andreev YG, Bruce PG (2003) Ionic conductivity in the crystalline polymer electrolytes PEO6:LiXF6, X = P, As, Sb. J Am Chem Soc 125:4619–4626
Tamman G, Hesse W (1926) The dependence of viscosity upon the temperature of supercooled liquids. Z Anorg Allg Chem 156:245–257
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Vogel H (1921) Temperature dependence of viscosity of melts. Z Phys 22:645–646
Wang YJ, Pan Y, Wang L, Pang MJ, Chen L (2006) Characterization of (PEO)LiClO4-Li1.3Al0.3Ti1.7(PO4)3 composite polymer electrolytes with different molecular weights of PEO. J Appl Poly Sci 102:4269–4275
Wang L, Yang W, Wang J, Evans DG (2009) New nanocomposite polymer electrolyte comprising nanosized ZnAl2O4 with a mesopore network and PEO–LiClO4. Solid State Ionics 180:392–397
Xi J, Tang X (2004) Nanocomposite polymer electrolyte based on poly(ethylene oxide) and solid super acid for lithium polymer battery. Chem Phys Lett 393:271–276
Xi J, Qiu X, Zheng S, Tang X (2005) Nanocomposite polymer electrolyte comprising PEO/LiClO4 and solid super acid: effect of sulphated-zirconia on the crystallization kinetics of PEO. Polymer 46:5702–5706
Xi J, Qiu X, Wang J, Bai Y, Zhu W, Chen L (2006) Effect of molecular sieves ZSM-5 on the crystallization behavior of PEO-based composite polymer electrolyte. J Power Sources 158:627–634
Acknowledgments
One (AK) of the authors acknowledges the Council of Scientific and Industrial Research, Government of India, for providing him research fellowship (via Grant no. 09/080(0573)/2007-EMR-I) for the work. The support by the Department of Science and Technology, Government of India through its nanoscience initiative program is also thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karmakar, A., Ghosh, A. Poly ethylene oxide (PEO)–LiI polymer electrolytes embedded with CdO nanoparticles. J Nanopart Res 13, 2989–2996 (2011). https://doi.org/10.1007/s11051-010-0194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0194-x