Abstract
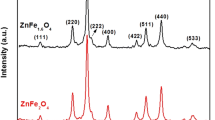
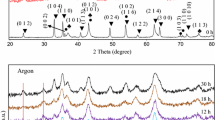
In this study, the synthesis of monophasic nanocrystalline zinc ferrite (ZnFe2O4) was achieved by controlling the thermal decomposition conditions of a zinc–iron tartrate precursor method. Differential thermal analysis/thermogravimetry (DTA/TG), X-ray diffraction (XRD), Fe2+ content analysis, transmission electron microscopy (TEM), and Brunauer-Emmett-Teller (BET) techniques were used to investigate the effect of heat treatment conditions on the calcined powders. The thermal decomposition of the precursor led to an intermediate phase formation of ZnO, Fe3O4, and γ-Fe2O3. It was found that the Fe3O4 → γ-Fe2O3 oxidation reaction is the key step in producing monophasic nanosized ZnFe2O4. The monophasic nanoparticles of ZnFe2O4 can be obtained when the precursor is heat treated under a low temperature (300–400 °C) and long residence time (4 h) process that can prompt the Fe3O4 oxidation and prevent the formation of α-Fe2O3.









Similar content being viewed by others
References
Braun PB (1952) A superstructure in spinels. Nature 170:1123
Can MM, Ozcan S, Firat T (2006) Magnetic behaviour of iron nanoparticles passivated by oxidation. Phys Status Solidi C: Conf 3:1271–1278
Druska P, Steinike U, Sepela’k V (1999) Surface structure of mechanically activated and of mechanosynthesized zinc ferrite. J Solid State Chem 146:13–21
Egger K, Feitknecht W (1962) Über die oxidation von Fe3O4 zu γ- und α-Fe2O3. Helv Chim Acta 45:2042–2057
Feitknecht W, Mannweiler U (1967) Der mechanismus der umwandlung vor γ- zu α-eisensesquioxid. Helv Chim Acta 50:570–581
Gajbhiye NS, Bhattacharya U, Darshane VS (1995) Thermal decomposition of zinc-iron citrate precursor. Thermochim Acta 264:219–230
Gillot B, Rousset A, Dupre G (1978) Influence of crystallite size on the oxidation kinetics of magnetite. J Solid State Chem 25:263–271
Gillot B, Benloucif RM, Rousset A (1981) A study of infrared absorption in the oxidation of zinc-substituted magnetites to defect phase γ and hematite. J Solid State Chem 39:329–336
Goss CJ (1988) Saturation magnetization, coercivity and lattice parameter changes in the system Fe3O4-γFe2O3, and their relationship to structure. Phys Chem Miner 16:164–171
Guaita FJ, Beltran H, Cordoncillo E, Beltran H, Escribano P, Gonzalez Calbet JM (1999) Influence of the precursors on the formation and the properties of ZnFe2O4. J Eur Ceram Soc 19:363–372
Hamdeh HH, Ho JC, Oliver SA, Willey RJ, Oliveri G, Busca G (1997) Magnetic properties of partially-inverted zinc ferrite aerogel powders. J Appl Phys 81:1851–1857
Hofmann M, Campbell SJ, Ehrhardt H, Feyerherm R (2004) The magnetic behaviour of nanostructured zinc ferrite. J Mater Sci 39:5057–5065
Jeyadevan B, Tohji K, Nakatsuka K (1994) Structure analysis of coprecipitated ZnFe2O4 by extended x-ray-absorption fine structure. J Appl Phys 76:6325–6327
Kamazawa K, Nakajima K, Kohn K, Tsunoda Y (2004) High magnetic field susceptibility and neutron scattering measurements for ZnFe2O4 single crystal. J Magn Magn Mater 272–276:e987–e988
Kester E, Perriat P, Gillot B, Tailhades Ph, Rousset A (1997) Correlation between oxidation states of transition metal ions and variation of coercivity in mixed-valence defect spinel ferrites. Solid State Ionics 101–103:457–463
Kim W, Saito F (2001) Mechanochemical synthesis of zinc ferrite from zinc oxide and α-Fe2O3. Powder Technol 114:12–16
Laarj M, Kacim S, Gillot B (1996) Cationic distribution and oxidation mechanism of trivalent manganese ions in submicrometer MnxCoFe2-xO4 spinel ferrites. J Solid State Chem 125:67–74
Lakeman CDE, Payne DA (1994) Sol–gel processing of electrical and magnetic ceramics. Mater Chem Phys 38:305–324
Li Y, Zhao J, He X (2004) Influence of oxygen pressure on combustion synthesis of zinc ferrite powders. Mater Sci Eng B 106:196–201
Mohai I, Szépvölgyi J, Bertóti I et al (2001) Thermal plasma synthesis of zinc ferrite nanopowders. Solid State Ionics 141–142:163–168
Morrison SA, Cahill CL, Carpenter EE, Calvin S, Harris VG (2003) Preparation and characterization of MnZn-ferrite nanoparticles using reverse micelles. J Appl Phys 93:7489–7491
Moye V, Rane KS, Kamat Dalal VN (1990) Optimization of synthesis of nickel-zinc-ferrite from oxalates and oxalato hydrazinate precursors. J Mater Sci Mater Electron 1:212–218
Nikumbh AK, Aware AD, Sayanekar PL (1992) Electrical and magnetic properties of γ-Fe2O3 synthesized from ferrous tartarate one and half hydrate. J Magn Magn Mater 114:27–34
Perriat P, Gillot B (1993) A model for coupled diffusion reactions in Mn-Zn ferrites—generalization of the Ficks’s first law. Solid State Ionics 67:35–43
Rane KS, Verenkar VMS, Sawant PY (1999) Hydrazine method of synthesis of γ-Fe2O3 useful in ferrites preparation. Part IV—preparation and characterization of magnesium ferrite, MgFe2O4 from γ-Fe2O3 obtained from hydrazinated iron oxyhydroxides and iron (II) carboxylatohydrazinates. J Mater Sci Mater Electron 10:133–140
Shenoy SD, Joy PA, Anantharaman MR (2004) Effect of mechanical milling on the structural, magnetic and dielectric properties of coprecipitated ultrafine zinc ferrite. J Magn Magn Mater 269:217–226
Sidhu PS (1988) Transformation of trace element-substituted maghemite to hematite. Clays Clay Miner 36:31–38
Sidhu PS, Gilkes RJ, Posner AM (1977) Mechanism of the low temperature oxidation of synthetic magnetites. J Inorg Nucl Chem 39:1953–1958
Swaddle TW, Oltmann P (1980) Kinetics of the magnetite-maghemite-hematite transformation, with special reference to hydrothermal systems. Can J Chem 58:1763–1772
Tamaura Y, Kodama T, Itoh T (1990) High-vacancy-content magnetites and zinc-bearing ferrites from iron (III) tartrate in strongly alkaline solution. J Am Ceram Soc 73:2539–2542
Vogel I (1961) A textbook of quantitative inorganic analysis. Longmans, New York, p 308
Willey RJ, Oliver SA, Oliveri G, Busca G (1993) Chemistry and structure of mixed magnesium ferric oxide aerogels. J Mater Res 8:1418–1427
Yang JM, Tsuo WJ, Yen FS (1999) Preparation of ultrafine nickel ferrite powders using mixed Ni and Fe tartrates. J Solid State Chem 145:50–57
Yang JM, Tsuo WJ, Yen FS (2001) Characterization of the thermal behavior of Li–Fe-tartrate gels (molar ratio Li/Fe<1/5). J Solid State Chem 160:100–107
Zhong W, Ding W, Zhang N, Hong J, Van Q, Du Y (1997) Key step in synthesis of ultrafine BaFe12O19 by sol-gel technique. J Magn Magn Mater 168:196–202
Acknowledgments
This work was sponsored by National Science Council of the Republic of China under Contract NSC 96-2622-E-244-001-CC3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J.M., Yang, K.L. An optimal low-temperature tartrate precursor method for the synthesis of monophasic nanosized ZnFe2O4 . J Nanopart Res 11, 1739–1750 (2009). https://doi.org/10.1007/s11051-008-9537-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-008-9537-2