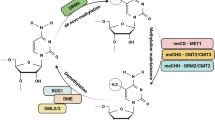
Abstract
Drought is an enormous threat to global crop production. In order to ensure food security for the burgeoning population, we must develop drought tolerant crop varieties. This necessitates the identification of drought-responsive genes and understanding the mechanisms involved in their regulation. DNA methylation is a widely studied mechanism of epigenetic regulation of gene expression, which is known to play vital role in conferring tolerance to various biotic and abiotic stress factors. The recent advances in next-generation sequencing (NGS) technologies, has allowed unprecedented access to genome-wide methylation marks, with single base resolution. The most important roles of DNA methylation have been studied in terms of gene body methylation (gbM), which is associated with regulation of both transcript abundance and its stability. The availability of mutants for the various genes encoding enzymes involved in methylation of DNA has allowed ascertainment of the biological significance of methylation. Even though a vast number of reports have emerged in the recent past, where both genome-wide methylation landscape and locus specific changes in DNA methylation have been studied, a conclusive picture with regards to the biological role of DNA methylation is still lacking. Compounding this, is the lack of sufficient evidence supporting the heritability of these epigenetic changes. Amongst the various epigenetic variations, the DNA methylation changes are observed to be the most stable. This review describes the drought-induced changes in DNA methylation identified across different plant species. We also briefly describe the stress memory contributed by these changes. The identification of heritable, drought-induced methylation marks would broaden the scope of crop improvement in the future.



Similar content being viewed by others
Data availability
All data analysed during this study are published articles.
References
Arias PA, Bellouin N, Coppola E, Masson-Delmotte V, Zhai P, Pirani A et al (2021) Summary for policymakers.. In: Climate Change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 3–32
Hendrawan VS, Komori D, Kim W (2023) Possible factors determining global-scale patterns of crop yield sensitivity to drought. PLoS ONE 18(2):e0281287
Sarkar B, Dutta S, Singh PK (2022) Drought and temporary migration in rural India: a comparative study across different socio-economic groups with a cross-sectional nationally representative dataset. PLoS ONE 17(10):e0275449. https://doi.org/10.1371/journal.pone.0275449
Gogoi A, Tripathi B (2019) https://www.indiaspend.com/42-indias-land-area-under-drought-worsening-farm-distress-in-election-year/
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop Production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Ahmad H, Li J (2021) Impact of water deficit on the development and senescence of tomato roots grown under various soil textures of Shaanxi, China. BMC Plant Biol 21:241. https://doi.org/10.1186/s12870-021-03018-1
Andleeb T, Shah T, Nawaz R, Munir I, Munsif F, Jalal A (2020) QTL mapping for drought stress tolerance in plants. In: Salt and drought stress tolerance in plants: signaling networks and adaptive mechanisms, pp 383–403
Sheoran S, Kaur Y, Kumar S, Shukla S, Rakshit S, Kumar R (2022) Recent advances for drought stress tolerance in maize (Zea mays L.): present status and future prospects. Front Plant Sci 13:872566. https://doi.org/10.3389/fpls.2022.872566
Pandey G, Sharma N, Sahu PP, Prasad M (2016) Chromatin-based epigenetic regulation of plant abiotic stress response. Curr Genomics 17(6):490–498. https://doi.org/10.2174/1389202917666160520103914
Kumar S, Mohapatra T (2021) Dynamics of DNA methylation and its functions in plant growth and development. Front Plant Sci 12:596236. https://doi.org/10.3389/fpls.2021.596236
Kakutani T, Munakata K, Richards EJ, Hirochika H (1999) Meiotically and mitotically stable inheritance of DNA hypomethylation induced by ddm1 mutation of Arabidopsis thaliana. Genetics 151:831–838
Papa CM, Springer NM, Muszynski MG, Meeley R, Kaeppler SM (2001) Maize chromomethylase Zea methyltransferase2 is required for CpNpG methylation. Plant Cell 13(8):1919–1928
Zhang H, Lang Z, Zhu JK (2018) Dynamics and function of DNA methylation in plants. Nat Rev Mol Cell Biol 19(8):489–506. https://doi.org/10.1038/s41580-018-0016-z
Gallego-Bartolomé J (2020) DNA methylation in plants: mechanisms and tools for targeted manipulation. New Phytol 227(1):38–44. https://doi.org/10.1111/nph.16529
Johnson MD, Mueller M, Game L, Aitman TJ (2012) Single nucleotide analysis of cytosine methylation by whole-genome shotgun bisulfite sequencing. Curr Protoc Mol Biol 99(1):21–23. https://doi.org/10.1002/0471142727.mb2123s99
Reyna-López GA, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 253:703–710. https://doi.org/10.1007/s004380050374
Singh KP (2014) Screening of DNA methylation changes by methylation-sensitive random amplified polymorphic DNA-polymerase chain reaction (MS-RAPD-PCR). Methods Mol Biol 1105:71–81. https://doi.org/10.1007/978-1-62703-739-6_6
Phutikanit N, Suwimonteerabutr J, Harrison D, D’Occhio M, Carroll B, Techakumphu M (2010) Different DNA methylation patterns detected by the Amplified Methylation Polymorphism Polymerase Chain Reaction (AMP PCR) technique among various cell types of bulls. Acta Vet Scand 52(1):18. https://doi.org/10.1186/1751-0147-52-18
The BLUEPRINT consortium (2016) Quantitative comparison of DNA methylation assays for biomarker development and clinical applications. Nat Biotechnol 34:726–737. https://doi.org/10.1038/nbt.3605
Kuo KC, McCune RA, Gehrke CW, Midgett R, Ehrlich M (1980) Quantitative reversed-phase high-performance liquid chromatographic determination of major and modified deoxyribonucleosides in DNA. Nucleic Acids Res 8:4763–4776. https://doi.org/10.1093/nar/8.20.4763
Quinlivan EP, Gregory JF (2008) DNA methylation determination by liquid chromatography–tandem mass spectrometry using novel biosynthetic [U-15N] deoxycytidine and [U-15N] methyldeoxycytidine internal standards. Nucleic Acids Res 36:e119. https://doi.org/10.1093/nar/gkn534
Flusberg BA, Webster DR, Lee JH, Travers KJ, Olivares EC, Clark TA, Korlach J, Turner SW (2010) Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods 7:461–465. https://doi.org/10.1038/nmeth.1459
Estécio MR, Gharibyan V, Shen L, Ibrahim AE, Doshi K, He R, Jelinek J, Yang AS, Yan PS, Huang TH, Tajara EH (2007) LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS ONE 2(5):e399. https://doi.org/10.1371/journal.pone.0000399
Mohn F, Weber M, Schübeler D, Roloff TC (2009) Methylated DNA immunoprecipitation (MeDIP). Methods Mol Biol 507:55–64. https://doi.org/10.1007/978-1-59745-522-0_5
Meissner A, Gnirke A, Bell GW, Ramsahoye B, Lander ES, Jaenisch R (2005) Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res 33:5868–5877. https://doi.org/10.1093/nar/gki901
Karimi M, Johanssona S, Stachc D, Corcorand M, Grandérd D, Schallingb M, Bakalkina G, Lykoc F, Larssonb C, Ekström TJ (2006) LUMA (LUminometric Methylation Assay)—a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res 312:1989–1995
Li Y, Tollefsbol TO (2011) DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 791:11–21. https://doi.org/10.1007/978-1-61779-316-5_2
Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB (1996) Methylation-specific pcr: a novel pcr assay for methylation status of cpg islands. Proc Natl Acad Sci USA 93:9821–9826. https://doi.org/10.1073/pnas.93.18.9821
Gonzalgo ML, Liang G (2007) Methylation-sensitive single-nucleotide primer extension (Ms-SNuPE) for quantitative measurement of DNA methylation. Nat Protoc 2(8):1931–1936. https://doi.org/10.1038/nprot.2007.271
Dahl C, Guldberg P (2003) DNA methylation analysis techniques. Biogerontology 4(4):233–250. https://doi.org/10.1023/a:1025103319328
Yokoyama S, Kitamoto S, Yamada N, Houjou I, Sugai T, Nakamura S, Arisaka Y, Takaori K, Higashi M, Yonezawa S (2012) The application of methylation specific electrophoresis (mse) to DNA methylation analysis of the 5′ cpg island of mucin in cancer cells. BMC Cancer 12:67. https://doi.org/10.1186/1471-2407-12-67
Xiong Z, Laird PW (1997) COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res 25(12):2532–2534. https://doi.org/10.1093/nar/25.12.2532
Lucibelli F, Valoroso MC, Aceto S (2022) Plant DNA methylation: an epigenetic mark in development, environmental interactions, and evolution. Int J Mol Sci 23(15):8299. https://doi.org/10.3390/ijms23158299
Agius DR, Kapazoglou A, Avramidou E, Baranek M, Carneros E, Caro E, Castiglione S, Cicatelli A, Radanovic A, Ebejer J-P, Gackowski D, Guarino F, Gulyás A, Hidvégi N, Hoenicka H, Inácio V, Johannes F, Karalija E, Lieberman-Lazarovich M, Martinelli F, Maury S, Mladenov V, Morais-Cecílio L, Pecinka A, Tani E, Testillano PS, Todorov D, Valledor L, Vassileva V (2023) Exploring the crop epigenome: a comparison of DNA methylation profiling techniques. Front Plant Sci 14:1181039. https://doi.org/10.3389/fpls.2023.1181039
Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL (1992) A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci 89(5):1827–1831. https://doi.org/10.1073/pnas.89.5.1827
Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452(7184):215–219. https://doi.org/10.1038/nature06745
Lei M, Zhang H, Julian R, Tang K, Xie S, Zhu JK (2015) Regulatory link between DNA methylation and active demethylation in Arabidopsis. Proc Natl Acad Sci USA 112(11):3553–3557. https://doi.org/10.1073/pnas.1502279112
Halpern BK, Vana T, Walker MD (2014) Paradoxical role of DNA methylation in activation of FoxA2 gene expression during endoderm development. J Biol Chem 289:23882–23892. https://doi.org/10.1074/jbc.M114.573469
Zhu H, Wang G, Qian J (2016) Transcription factors as readers and effectors of DNA methylation. Nat Rev Genet 17:551–565. https://doi.org/10.1038/nrg.2016.83
Bewick AJ, Schmitz RJ (2017) Gene body DNA methylation in plants. Curr Opin Plant Biol. https://doi.org/10.1016/j.pbi.2016.12.007
Muyle AM, Seymour DK, Huettel B, Lv Y, Gaut BS (2022) Gene body methylation in plants: mechanisms, functions, and important implications for understanding evolutionary processes. Genome Biol Evol 14(4):038
Horvath R, Laenen B, Takuno S, Slotte T (2019) Single-cell expression noise and gene-body methylation in Arabidopsis thaliana. Heredity 123(2):81–91. https://doi.org/10.1038/s41437-018-0181-z
Shahzad Z, Moore JD, Zilberman D (2021) Gene body methylation mediates epigenetic inheritance of plant traits. bioRxiv. 2021.03.15.435374
Li Q, Chen S, Leung AW-S, Liu Y, Xin Y et al (2021) DNA methylation affects pre-mRNA transcriptional initiation and processing in Arabidopsis. Biorxiv. https://doi.org/10.1101/2021.04.29.441938
Bewick AJ, Ji L, Niederhuth CE, Willing E-M, Hofmeister BT et al (2016) On the origin and evolutionary consequences of gene body DNA methylation. Proc Natl Acad Sci USA 113:9111–9116
Huang J, Wang Y, Yu J, Li F, Yi L, Li Y, Xie N, Wu Q, Samarina L, Tong W, Xia E (2023) Evolutionary landscape of tea circular RNAs and its contribution to chilling tolerance of tea plant. Int J Mol Sci 24(2):1478. https://doi.org/10.3390/ijms24021478
Rigal M, Becker C, Pélissier T, Pogorelcnik R, Devos J, Ikeda Y, Weigel D, Mathieu O (2016) Epigenome confrontation triggers immediate reprogramming of DNA methylation and transposon silencing in Arabidopsis thaliana F1 epihybrids. Proc Natl Acad Sci USA 113(14):E2083–E2092. https://doi.org/10.1073/pnas.1600672113
To TK, Kim JM (2014) Epigenetic regulation of gene responsiveness in Arabidopsis. Front Plant Sci 4:548. https://doi.org/10.3389/fpls.2013.00548
Ong-Abdullah M, Ordway JM, Jiang N, Ooi SE, Kok SY, Sarpan N, Azimi N, Hashim AT, Ishak Z, Rosli SK, Malike FA (2015) Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature 525(7570):533–537
Zhang Z, Wang H, Wang Y, Xi F, Wang H, Kohnen MV, Gao P, Wei W, Chen K, Liu X, Gao Y (2021) Whole-genome characterization of chronological age-associated changes in methylome and circular RNAs in moso bamboo (Phyllostachys edulis) from vegetative to floral growth. Plant J 106(2):435–453. https://doi.org/10.1111/tpj.15174
Feng S, Cokus SJ, Schubert V, Zhai J, Pellegrini M, Jacobsen SE (2014) Genome-wide Hi-C analyses in wild-type and mutants reveal high-resolution chromatin interactions in Arabidopsis. Mol Cell 55(5):694–707
Tsukahara S, Kobayashi A, Kawabe A, Mathieu O, Miura A, Kakutani T (2009) Bursts of retrotransposition reproduced in Arabidopsis. Nature 461(7262):423–426
Wang WS, Pan YJ, Zhao XQ, Dwivedi D, Zhu LH, Ali J, Fu BY, Li ZK (2011) Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J Exp Bot 62:1951–1960
Zheng X, Chen L, Li M, Lou Q, Xia H, Wang P, Li T, Liu H, Luo L (2013) Transgenerational variations in DNA methylation induced by drought stress in two rice varieties with distinguished difference to drought resistance. PLoS ONE 8(11):e80253. https://doi.org/10.1371/journal.pone.0080253
Garg R, Narayana Chevala VV, Shankar R, Jain M (2015) Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci Rep 5(1):14922. https://doi.org/10.1038/srep14922
Wang W, Qin Q, Sun F, Wang Y, Xu D, Li Z, Fu B (2016) Genome-wide differences in DNA methylation changes in two contrasting rice genotypes in response to drought conditions. Front Plant Sci 7:1675
Sapna H, Ashwini N, Ramesh S, Nataraja KN (2020) Assessment of DNA methylation pattern under drought stress using methylation-sensitive randomly amplified polymorphism analysis in rice. Plant Genet Resour 18(4):222–230
Rajkumar MS, Shankar R, Garg R, Jain M (2020) Bisulphite sequencing reveals dynamic DNA methylation under desiccation and salinity stresses in rice cultivars. Genomics 112(5):3537–3548
Li P, Yang H, Wang L, Liu H, Huo H, Zhang C, Liu A, Zhu A, Hu J, Lin Y, Liu L (2019) Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front Genet 10:55
Kou S, Gu Q, Duan L, Liu G, Yuan P, Li H, Wu Z, Liu W, Huang P, Liu L (2022) Genome-wide bisulphite sequencing uncovered the contribution of DNA methylation to rice short-term drought memory formation. J Plant Growth Regul 41(7):2903–2917
Ding G, Cao L, Zhou J, Li Z, Lai Y, Liu K, Luo Y, Bai L, Wang X, Wang T, Wang R (2022) DNA methylation correlates with the expression of drought-responsive genes and drought resistance in rice. Agronomy 12(6):1445
Tan MP (2009) Analysis of DNA methylation of maize in response to osmotic and salt stress based on methylation-sensitive amplified polymorphism. Plant Physiol Biochem 48(1):21–26. https://doi.org/10.1016/j.plaphy.2009.10.005
Sallam N, Moussa M, Yacout M, El-Seedy A (2019) Differential DNA methylation under drought stress in maize. Int J Curr Microbiol Appl Sci 8:2527–2543
Sallam N, Moussa M, Yacout M, El-Seedy A (2020) Analysis of methylated genomic cytosines of maize inbred line W22 in response to drought stress. Cereal Res Commun 48:459–465
Wang Q, Xu J, Pu X, Lv H, Liu Y, Ma H, Wu F, Wang Q, Feng X, Liu T, Tang Q (2021) Maize DNA methylation in response to drought stress is involved in target gene expression and alternative splicing. Int J Mol Sci 22(15):8285
Surdonja K, Eggert K, Hajirezaei MR, Harshavardhan VT, Seiler C, Von Wirén N, Sreenivasulu N, Kuhlmann M (2017) Increase of DNA methylation at the HvCKX2. 1 promoter by terminal drought stress in barley. Epigenomes 1(2):9
Drosou V, Kapazoglou A, Letsiou S, Tsaftaris AS, Argiriou A (2021) Drought induces variation in the DNA methylation status of the barley HvDME promoter. J Plant Res 134:1351–1362
Falahi A, Zarei L, Cheghamirza K (2022) Most drought-induced DNA methylation changes switched to pre-stress state after re-irrigation in barley (Hordeum vulgare L.) cultivars. Cereal Res. Commun. 50(3):429–38
Hubbard M, Germida JJ, Vladimir V (2014) Fungal endophyte colonization coincides with altered DNA methylation in drought-stressed wheat seedlings. Can J Plant Sci 94(2):223–234
Kaur A, Grewal A, Sharma P (2018) Comparative analysis of DNA methylation changes in two contrasting wheat genotypes under water deficit. Biol Plant 62:471–478
Ganguly DR, Crisp PA, Eichten SR, Pogson BJ (2017) The Arabidopsis DNA methylome is stable under transgenerational drought stress. Plant Physiol 175(4):1893–1912
Van Dooren TJ, Silveira AB, Gilbault E, Jiménez-Gómez JM, Martin A, Bach L, Tisné S, Quadrana L, Loudet O, Colot V (2020) Mild drought in the vegetative stage induces phenotypic, gene expression, and DNA methylation plasticity in Arabidopsis but no transgenerational effects. J Exp Bot 71(12):3588–3602
Fan H, Li T, Guan L, Li Z, Guo N, Cai Y, Lin Y (2012) Effects of exogenous nitric oxide on antioxidation and DNA methylation of Dendrobium huoshanense grown under drought stress. Plant Cell Tissue Org Cult 109:307–314. https://doi.org/10.1007/s11240-011-0096-3
Li Y, Cheng P, Xiong G, Hong Y (2012) Studies on DNA methylation in potato under drought stress. Chin Potato J 26(1):11–16
Bhardwaj J, Mahajan M, Yadav SK (2013) Comparative analysis of DNA methylation polymorphism in drought-sensitive (HPKC2) and tolerant (HPK4) genotypes of horse Gram (Macrotyloma uniflorum). Biochem Genet 51(7–8):493–502. https://doi.org/10.1007/s10528-013-9580-2
Liang D, Zhang Z, Wu H, Huang C, Shuai P, Ye CY, Tang S, Wang Y, Yang L, Wang J, Yin W (2014) Single-base-resolution methylomes of Populus trichocarpa reveal the association between DNA methylation and drought stress. BMC Genet 15(1):1–11. https://doi.org/10.1186/1471-2156-15-S1-S9
Tang XM, Tao X, Wang Y, Ma DW, Li D, Yang H, Ma XR (2014) Analysis of DNA methylation of perennial ryegrass under drought using the methylation-sensitive amplification polymorphism (MSAP) technique. Mol Genet Genomics 289:1075–1084
Correia B, Valledor L, Hancock RD, Jesus C, Amaral J, Meijon M, Pinto G (2016) Depicting how Eucalyptus globulus survives drought: involvement of redox and DNA methylation events. Funct Plant Biol 43(9):838–850
Abid G, Mingeot D, Muhovski Y, Mergeai G, Aouida M, Abdelkarim S, Aroua I, El Ayed M, M’hamdi M, Sassi K, Jebara M (2017) Analysis of DNA methylation patterns associated with drought stress response in faba bean (Vicia faba L.) using methylation-sensitive amplification polymorphism (MSAP). Environ Exp Bot 142:34–44
Lu X, Wang X, Chen X, Shu N, Wang J, Wang D, Wang S, Fan W, Guo L, Guo X, Ye W (2017) Single-base resolution methylomes of upland cotton (Gossypium hirsutum L.) reveal epigenome modifications in response to drought stress. BMC Genomics 18(1):1–4
Komivi D, Marie AM, Rong Z, Qi Z, Mei Y, Ndiaga C, Diaga D, Linhai W, Xiurong Z (2018) The contrasting response to drought and waterlogging is underpinned by divergent DNA methylation programs associated with transcript accumulation in sesame. Plant Sci 277:207–217. https://doi.org/10.1016/j.plantsci.2018.09.012
Al-Harrasi I, Al-Yahyai R, Yaish MW (2018) Differential DNA methylation and transcription profiles in date palm roots exposed to salinity. PLoS ONE 13(1):e0191492. https://doi.org/10.1371/journal.pone.0191492
Yuan Y, Zhu S, Fang T, Jiang J, Wang Y (2019) Analysis of drought resistance and DNA methylation level of resynthesized Brassica napus. Acta Agron Sin 45(5):693–704
De Kort H, Panis B, Deforce D, Van Nieuwerburgh F, Honnay O (2020) Ecological divergence of wild strawberry DNA methylation patterns at distinct spatial scales. Mol Ecol 29(24):4871–4881
Hao X, Jin Z, Wang Z, Qin W, Pei Y (2020) Hydrogen sulfide mediates DNA methylation to enhance osmotic stress tolerance in Setaria italica L. Plant Soil 453:355–370
Ackah M, Jin X, Zhang Q, Amoako FK, Wang L, Attaribo T, Zhao M, Yuan F, Herman RA, Qiu C, Lin Q (2022) Long noncoding RNA transcriptome analysis reveals novel lncRNAs in Morus alba ‘Yu‐711’response to drought stress. Plant Genome e20273
Lyu Z, Zhang G, Song Y, Diao S, He C, Zhang J (2022) Transcriptome and DNA methylome provide insights into the molecular regulation of drought stress in sea buckthorn. Genomics 114(3):110345
Zhou J, Song F, He Y (2023) LncRNA evolution and DNA methylation variation participate in photosynthesis pathways of distinct lineages of Populus. Forestry Res 3(1)
Niu C, Jiang L, Cao F, Liu C, Guo J, Zhang Z, Yue Q, Hou N, Liu Z, Li X, Tahir MM, He J, Li Z, Li C, Ma F, Guan Q (2022) Methylation of a MITE insertion in the MdRFNR1-1 promoter is positively associated with its allelic expression in apple in response to drought stress. Plant Cell 34(10):3983–4006. https://doi.org/10.1093/plcell/koac220
Zhao P, Ma B, Cai C, Xu J (2022) Transcriptome and methylome changes in two contrasting mungbean genotypes in response to drought stress. BMC Genomics 23(1):80
Luo D, Cao S, Li Z, Tang M, Wang C, Mackon E, Huang Z, Pan J, Wu X, Wu Q, Zhang H (2022) Methyl-sensitive amplification polymorphism (MSAP) analysis provides insights into the DNA methylation underlying heterosis in Kenaf (Hibiscus cannabinus L.) drought tolerance. J. Nat. Fibers 19(16):13665–80
Wang L, Wang L, Tan M, Wang L, Zhao W, You J, Wang L, Yan X, Wang W (2023) The pattern of alternative splicing and DNA methylation alteration and their interaction in linseed (Linum usitatissimum L.) response to repeated drought stresses. Biol Res 56(1):1–6
González RM, Ricardi MM, Iusem ND (2013) Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics 8(8):864–872. https://doi.org/10.4161/epi.25524
Santos AP, Ferreira LJ, Oliveira MM (2017) Concerted flexibility of chromatin structure, methylome, and histone modifications along with plant stress responses. Biology 6(1):3
Zhu C, Zhang S, Zhou C, Chen L, Fu H, Li X, Lin Y, Lai Z, Guo Y (2020) Genome-wide investigation and transcriptional analysis of cytosine-5 DNA methyltransferase and DNA demethylase gene families in tea plant (Camellia sinensis) under abiotic stress and withering processing. Peer J 8:e8432
Yaish MW (2013) DNA methylation-associated epigenetic changes in stress tolerance of plants. In: Molecular stress physiology of plants. Springer, India, pp 427–440
Sow MD, Le Gac AL, Fichot R, Lanciano S, Delaunay A, Le Jan I, Lesage-Descauses MC, Citerne S, Caius J, Brunaud V, Soubigou-Taconnat L, Cochard H, Segura V, Chaparro C, Grunau C, Daviaud C, Tost J, Brignolas F, Strauss SH, Mirouze M, Maury S (2021) RNAi suppression of DNA methylation affects the drought stress response and genome integrity in transgenic poplar. New Phytol 232(1):80–97. https://doi.org/10.1111/nph.17555
Tian W et al (2021) SDC mediates DNA methylation-controlled clock pace by interacting with ZTL in Arabidopsis. Nucleic Acids Res 49:3764–3780
He L, Huang H, Bradai M et al (2022) DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development. Nat Commun 13:1335. https://doi.org/10.1038/s41467-022-28940-2
Cao X, Jacobsen SE (2002) Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12(13):1138–1144. https://doi.org/10.1016/s0960-9822(02)00925-9
Zhou M, Palanca AM, Law JA (2018) Locus-specific control of the de novo DNA methylation pathway in Arabidopsis by the CLASSY family. Nat Genet 50(6):865–873
Zhou M, Coruh C, Xu G, Martins LM, Bourbousse C, Lambolez A, Law JA (2022) The CLASSY family controls tissue-specific DNA methylation patterns in Arabidopsis. Nat Commun 13(1):244
Castano-Duque L, Ghosal S, Quilloy FA, Mitchell-Olds T, Dixit S (2021) An epigenetic pathway in rice connects genetic variation to anaerobic germination and seedling establishment. Plant Physiol 186(2):1042–1059. https://doi.org/10.1093/plphys/kiab100
Fleta-Soriano E, Munné-Bosch S (2016) Stress memory and the inevitable effects of drought: a physiological perspective. Front Plant Sci 7:143. https://doi.org/10.3389/fpls.2016.00143
Ramakrishnan M, Zhang Z, Mullasseri S, Kalendar R, Ahmad Z, Sharma A, Liu G, Zhou M, Wei Q (2022) Epigenetic stress memory: a new approach to study cold and heat stress responses in plants. Front Plant Sci 13:1075279. https://doi.org/10.3389/fpls.2022.1075279
Rendina González AP, Dumalasová V, Rosenthal J, Skuhrovec J, Latzel V (2017) The role of transgenerational effects in adaptation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol Ecol 31:345. https://doi.org/10.1007/s10682-016-9844-5
Zheng X, Chen L, Li M, Lou Q, Xia H, Wang P et al (2013) Transgenerational variations in DNA methylation induced by drought stress in two rice varieties with distinguished difference to drought resistance. PLoS ONE 8:e80253. https://doi.org/10.1371/journal.pone.0080253
Baránek M, Čechová J, Raddová J, Holleinová V, Ondrušíková E, Pidra M (2015) Dynamics and reversibility of the DNA methylation landscape of grapevine plants (Vitis vinifera) stressed by in vitro cultivation and thermotherapy. PLoS ONE 10(5):e0126638. https://doi.org/10.1371/journal.pone.0126638
Lämke J, Bäurle I (2017) Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol 18(1):124. https://doi.org/10.1186/s13059-017-1263-6
Zong W, Zhong X, You J, Xiong L (2013) Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Mol Biol 81(1–2):175–188
Fang H, Liu X, Thorn G, Duan J, Tian L (2014) Expression analysis of histone acetyl transferases in rice under drought stress. Biochem Biophys Res Commun 443:400–405
Acknowledgements
The authors would like to thank the Director, ICAR-NIPB for providing valuable guidance during the course of study for the first author.
Funding
The authors work in this area is supported by fellowship provided by NAHEP-CAAST to SY and DST- SERB (CRG/2019/006643) Grant to PKJ.
Author information
Authors and Affiliations
Contributions
SY and PKJ conceived the idea and prepared the draft manuscript. GK and SM suggested improvements in the article. All authors approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, S., Meena, S., Kalwan, G. et al. DNA methylation: an emerging paradigm of gene regulation under drought stress in plants. Mol Biol Rep 51, 311 (2024). https://doi.org/10.1007/s11033-024-09243-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09243-9