Abstract
Background
Ovarian advanced glycation end-products (AGEs) accumulation is associated with ovarian granulosa cells (GCs) dysfunction. Vitamin B6 derivatives positively affected reproduction. The current study was conducted to elucidate the AGEs effects on human luteinized mural GCs steroidogenesis in the presence or absence of pyridoxamine (PM).
Methods and results
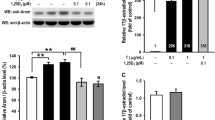
Isolated GCs of 50 healthy women were divided into four parts and treated with media alone (Control), PM alone, or human glycated albumin (HGA) with/without PM. Main steroidogenic enzymes and hormones were assessed by qRT-PCR and ELISA. The AGE receptor (RAGE) protein was also determined using Western blotting. The non-toxic concentration of HGA increased the expression of RAGE, StAR, 3β-HSD, and 17β-HSD (P < 0.0001 for all) but decreased the expression of CYP19A1 at mRNA levels. The increased RAGE protein expression was also confirmed by western blot analysis. These effects resulted in declined estradiol (E2), slightly, and a sharp rise in progesterone (P4) and testosterone (T) levels, respectively. PM, on its own, ameliorated the HGA-altered enzyme expression and, thereby, corrected the aberrant levels of E2, P4, and T. These effects are likely mediated by regulating the RAGE gene and protein expression.
Conclusion
This study indicates that hormonal dysfunctions induced by the AGEs-RAGE axis in luteinized GCs are likely rectified by PM treatment. This effect is likely acquired by reduced expression of RAGE. A better understanding of how AGEs and PM interact in ovarian physiology and pathology may lead to more targeted therapy for treating ovarian dysfunction.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Andersen CY, Ezcurra D (2014) Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod Biol Endocrinol 12:128. https://doi.org/10.1186/1477-7827-12-128
Hurst BS, Merriam KS, Elliot M, Matthews ML, Marshburn PB, Usadi RS, Hurst BS (2015) A sustained elevated estradiol is not the trigger for the pre-ovulatory luteinizing hormone surge. Women’s Health Gynecol 3:10–18
Dozortsev DI, Pellicer A, Diamond MP (2020) Premature progesterone rise as a trigger of polycystic ovarian syndrome. Fertil Steril 114:943–944. https://doi.org/10.1016/j.fertnstert.2020.07.007
Dozortsev DI, Diamond MP (2020) Luteinizing hormone-independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil Steril 114:191–199. https://doi.org/10.1016/j.fertnstert.2020.06.016
Venetis CA, Storr A, Chua SJ, Mol BW, Longobardi S, Yin X, D’Hooghe T (2023) What is the optimal GnRH antagonist protocol for ovarian stimulation during ART treatment? A systematic review and network meta-analysis. Hum Reprod Update. https://doi.org/10.1093/humupd/dmac040
Lawrenz B, Melado L, Fatemi H (2018) Premature progesterone rise in ART-cycles. Reprod Biol 18:1–4
Shen C-Y, Lu C-H, Wu C-H, Li K-J, Kuo Y-M, Hsieh S-C, Yu C-L (2020) The development of Maillard reaction, and Advanced Glycation End product (AGE)-Receptor for AGE (RAGE) signaling inhibitors as Novel therapeutic strategies for patients with AGE-Related Diseases. Molecules 25:5591
Prasad C, Davis KE, Imrhan V, Juma S, Vijayagopal P (2019) Advanced Glycation End Products and Risks for Chronic Diseases: intervening through Lifestyle Modification. Am J Lifestyle Med 13:384–404. https://doi.org/10.1177/1559827617708991
Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M (2022) Advanced Glycation End-Products (AGEs): formation, Chemistry, classification, receptors, and Diseases related to AGEs. Cells 11. https://doi.org/10.3390/cells11081312
Bongarzone S, Savickas V, Luzi F, Gee AD (2017) Targeting the receptor for Advanced Glycation End-products (RAGE): a Medicinal Chemistry Perspective. J Med Chem 60:7213–7232. https://doi.org/10.1021/acs.jmedchem.7b00058
Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, Kazeroni M (2020) Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic anti-oxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol 197:105521. https://doi.org/10.1016/j.jsbmb.2019.105521
Mehdinejadiani S, Amidi F, Mehdizadeh M, Barati M, Safdarian L, Aflatoonian R, Alyasin A, Aghahosseini M, Pazhohan A, Hayat P, Mohammadzadeh Kazorgah F, Sobhani A (2018) The effects of letrozole and clomiphene citrate on ligands expression of Wnt3, Wnt7a, and Wnt8b in proliferative endometrium of women with polycystic ovarian syndrome. Gynecol Endocrinol 34:775–780. https://doi.org/10.1080/09513590.2018.1446934
Merhi Z, Kandaraki EA, Diamanti-Kandarakis E (2019) Implications and future perspectives of AGEs in PCOS Pathophysiology. Trends Endocrinol Metab 30:150–162. https://doi.org/10.1016/j.tem.2019.01.005
Stensen MH, Tanbo T, Storeng R, Fedorcsak P (2014) Advanced glycation end products and their receptor contribute to ovarian ageing. Hum Reprod 29:125–134. https://doi.org/10.1093/humrep/det419
Garg D, Merhi Z (2016) Relationship between Advanced Glycation End Products and Steroidogenesis in PCOS. Reprod Biol Endocrinol 14:71. https://doi.org/10.1186/s12958-016-0205-6
Diamanti-Kandarakis E, Chatzigeorgiou A, Papageorgiou E, Koundouras D, Koutsilieris M (2016) Advanced glycation end-products and insulin signaling in granulosa cells. Exp Biol Med 241:1438–1445
Merhi Z, Buyuk E, Cipolla M (2018) Advanced glycation end products alter steroidogenic gene expression by granulosa cells: an effect partially reversible by vitamin D. Mol Hum Reprod 24:318–326
Takahashi N, Harada M, Azhary JM, Kunitomi C, Nose E, Terao H, Koike H, Wada-Hiraike O, Hirata T, Hirota Y (2019) Accumulation of advanced glycation end products in follicles is associated with poor oocyte developmental competence. Mol Hum Reprod 25:684–694
Chen JL, Francis J (2012) Pyridoxamine, advanced glycation inhibition, and diabetic nephropathy. J Am Soc Nephrol 23:6–8. https://doi.org/10.1681/asn.2011111097
Ramis R, Ortega-Castro J, Caballero C, Casasnovas R, Cerrillo A, Vilanova B, Adrover M, Frau J (2019) How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Anti-oxidant Activity. Anti-oxidants (Basel) 8. https://doi.org/10.3390/antiox8090344
Lyon P, Strippoli V, Fang B, Cimmino L (2020) B vitamins and One-Carbon Metabolism: implications in Human Health and Disease. Nutrients 12. https://doi.org/10.3390/nu12092867
Glaeser JD, Ju D, Tawackoli W, Yang JH, Salehi K, Stefanovic T, Kanim LEA, Avalos P, Kaneda G, Stephan S, Metzger MF, Bae HW, Sheyn D (2020) Advanced glycation end product inhibitor pyridoxamine attenuates IVD degeneration in type 2 Diabetic rats. Int J Mol Sci 21. https://doi.org/10.3390/ijms21249709
Aboelenain M, Balboula AZ, Kawahara M, El-Monem Montaser A, Zaabel SM, Kim SW, Nagano M, Takahashi M (2017) Pyridoxine supplementation during oocyte maturation improves the development and quality of bovine preimplantation embryos. Theriogenology 91:127–133. https://doi.org/10.1016/j.theriogenology.2016.12.022
Chen X, Lu T, Wang X, Sun X, Zhang J, Zhou K, Ji X, Sun R, Wang X, Chen M, Ling X (2020) Metabolic alterations associated with polycystic ovary syndrome: a UPLC Q-Exactive based metabolomic study. Clin Chim Acta 502:280–286. https://doi.org/10.1016/j.cca.2019.11.016
Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, Haydardedeoglu B, Kuscu E (2005) Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod 20:1521–1528. https://doi.org/10.1093/humrep/deh825
Hestiantoro A, Astuti BPK, Joyo EO, Febri RR, Silvana V, Muharam R (2022) Vitamin B(3) (niacin), B(6), C, and iron intake are associated with the free androgen index, especially in normoandrogenic polycystic ovary syndrome. J Turk Ger Gynecol Assoc 23:130–136. https://doi.org/10.4274/jtgga.galenos.2022.2022-2-1
Chen X, Thibeault S (2013) Effect of DMSO concentration, cell density and needle gauge on the viability of cryopreserved cells in three dimensional hyaluronan hydrogel. Annu Int Conf IEEE Eng Med Biol Soc 2013:6228–6231. https://doi.org/10.1109/embc.2013.6610976
Tatone C, Di Emidio G, Placidi M, Rossi G, Ruggieri S, Taccaliti C, D’Alfonso A, Amicarelli F, Guido M (2021) AGEs-related dysfunctions in PCOS: evidence from animal and clinical research. J Endocrinol 251:R1–r9. https://doi.org/10.1530/joe-21-0143
Mouanness M, Merhi Z (2022) Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways. Nutrients [serial on the Internet]. ; 14(5)
Garg D, Merhi Z (2015) Advanced glycation end products: link between diet and ovulatory dysfunction in PCOS? Nutrients 7:10129–10144
Pertynska-Marczewska M, Diamanti-Kandarakis E (2017) Aging ovary and the role for advanced glycation end products. Menopause 24:345–351
Wang X, Wang L, Xiang W (2023) Mechanisms of ovarian aging in women: a review. J Ovarian Res 16:67. https://doi.org/10.1186/s13048-023-01151-z
Merhi Z, Irani M, Doswell AD, Ambroggio J (2014) Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): a potential indicator of ovarian reserve. J Clin Endocrinol Metab 99:E226–E233
Zhu J-l, Cai Y-q, Long S-l, Chen Z, Mo Z-c (2020) The role of advanced glycation end products in human infertility. Life Sci 255:117830. https://doi.org/10.1016/j.lfs.2020.117830
Ravichandran G, Lakshmanan DK, Raju K, Elangovan A, Nambirajan G, Devanesan AA, Thilagar S (2019) Food advanced glycation end products as potential endocrine disruptors: an emerging threat to contemporary and future generation. Environ Int 123:486–500. https://doi.org/10.1016/j.envint.2018.12.032
Niu G, Guo J, Tian Y, Zhao K, Li J, Xiao Q (2018) α–lipoic acid can greatly alleviate the toxic effect of AGES on SH–SY5Y cells. Int J Mol Med 41:2855–2864
Prantner D, Nallar S, Vogel SN (2020) The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways. FASEB J 34:15659–15674. https://doi.org/10.1096/fj.202002136R
Merhi Z, Du XQ, Charron MJ (2020) Perinatal exposure to high dietary advanced glycation end products affects the reproductive system in female offspring in mice. Mol Hum Reprod 26:615–623
Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, Ai J, Jin L (2016) Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS ONE 11:e0145895
Kandaraki EA, Chatzigeorgiou A, Papageorgiou E, Piperi C, Adamopoulos C, Papavassiliou AG, Koutsilieris M, Diamanti-Kandarakis E (2018) Advanced glycation end products interfere in luteinizing hormone and follicle stimulating hormone signaling in human granulosa KGN cells. Exp Biol Med (Maywood) 243:29–33. https://doi.org/10.1177/1535370217731288
Dompe C, Kulus M, Stefańska K, Kranc W, Chermuła B, Bryl R, Pieńkowski W, Nawrocki MJ, Petitte JN, Stelmach B, Mozdziak P, Jeseta M, Pawelczyk L, Jaśkowski JM, Piotrowska-Kempisty H, Spaczyński RZ, Nowicki M, Kempisty B (2021) Human granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. https://doi.org/10.3390/cells10061396. Cells 10
Gao EM, Turathum B, Wang L, Zhang D, Liu YB, Tang RX, Chian RC (2022) The Differential Metabolomes in Cumulus and Mural Granulosa cells from human pre-ovulatory follicles. Reprod Sci 29:1343–1356. https://doi.org/10.1007/s43032-021-00691-3
Turgut F, Bolton WK (2010) Potential New Therapeutic Agents for Diabetic kidney disease. Am J Kidney Dis 55:928–940. https://doi.org/10.1053/j.ajkd.2009.11.021
Steegers-Theunissen RP, Steegers EA, Thomas CM, Hollanders HM, Peereboom-Stegeman JH, Trijbels FJ, Eskes TK (1993) Study on the presence of homocysteine in ovarian follicular fluid. Fertil Steril 60:1006–1010. https://doi.org/10.1016/s0015-0282(16)56401-2
Allgood VE, Cidlowski JA (1992) Vitamin B6 modulates transcriptional activation by multiple members of the steroid hormone receptor superfamily. J Biol Chem 267:3819–3824
Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC (2008) Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertil Steril 89:668–676. https://doi.org/10.1016/j.fertnstert.2007.03.089
Govahi A, Amjadi F, Nasr-Esfahani MH, Raoufi E, Mehdizadeh M (2022) Accompaniment of Time-Lapse Parameters and Cumulus Cell RNA-Sequencing in embryo evaluation. Reprod Sci 29:395–409. https://doi.org/10.1007/s43032-021-00748-3
Zhao Y, Hu S, Zhai W, Wang M, Ran L (2022) Clinical study of Progesterone combined with vitamin B6 in the treatment of Amenorrhea Endocrine Disorders caused by antipsychotics. Comput Math Methods Med 2022:2436322. https://doi.org/10.1155/2022/2436322
Szczuko M, Hawryłkowicz V, Kikut J, Drozd A (2020) The implications of vitamin content in the plasma in reference to the parameters of carbohydrate metabolism and hormone and lipid profiles in PCOS. J Steroid Biochem Mol Biol 198:105570. https://doi.org/10.1016/j.jsbmb.2019.105570
Scammahorn JJ, Nguyen ITN, Bos EM, Van Goor H, Joles JA (2021) Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Anti-oxidants (Basel) 10. https://doi.org/10.3390/antiox10030373
Yang J, Minkler P, Grove D, Wang R, Willard B, Dweik R, Hine C (2019) Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun Biol 2:194. https://doi.org/10.1038/s42003-019-0431-5
Golestanfar A, Niasari-Naslaji A, Jafarpour F, Rouhollahi S, Rezaei N, Menezo Y, Dattilo M, Nasr-Esfahani MH (2022) Metabolic enhancement of the one carbon metabolism (OCM) in bovine oocytes IVM increases the blastocyst rate: evidences for a OCM checkpoint. Sci Rep 12:20629. https://doi.org/10.1038/s41598-022-25083-8
Ueland PM, Ulvik A, Rios-Avila L, Midttun Ø, Gregory JF (2015) Direct and functional biomarkers of vitamin B6 status. Annu Rev Nutr 35:33–70. https://doi.org/10.1146/annurev-nutr-071714-034330
Furness D, Fenech M, Dekker G, Khong TY, Roberts C, Hague W (2013) Folate, vitamin B12, vitamin B6 and homocysteine: impact on pregnancy outcome. Matern Child Nutr 9:155–166. https://doi.org/10.1111/j.1740-8709.2011.00364.x
Deepa R, Mandal S, Van Schayck OCP, Babu GR (2023) Vitamin B6 levels and impaired Folate Status but not vitamin B12 Associated with Low Birth Weight: results from the MAASTHI Birth Cohort in South India. Nutrients 15. https://doi.org/10.3390/nu15071793
Ronnenberg AG, Goldman MB, Chen D, Aitken IW, Willett WC, Selhub J, Xu X (2002) Preconception homocysteine and B vitamin status and birth outcomes in chinese women. Am J Clin Nutr 76:1385–1391. https://doi.org/10.1093/ajcn/76.6.1385
Ronnenberg AG, Venners SA, Xu X, Chen C, Wang L, Guang W, Huang A, Wang X (2007) Preconception B-vitamin and homocysteine status, conception, and early pregnancy loss. Am J Epidemiol 166:304–312. https://doi.org/10.1093/aje/kwm078
Goddijn-Wessel TA, Wouters MG, van de Molen EF, Spuijbroek MD, Steegers-Theunissen RP, Blom HJ, Boers GH, Eskes TK (1996) Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur J Obstet Gynecol Reprod Biol 66:23–29. https://doi.org/10.1016/0301-2115(96)02383-4
Wouters MG, Boers GH, Blom HJ, Trijbels FJ, Thomas CM, Borm GF, Steegers-Theunissen RP, Eskes TK (1993) Hyperhomocysteinemia: a risk factor in women with unexplained recurrent early pregnancy loss. Fertil Steril 60:820–825
Bjørke-Monsen AL, Varsi K, Sakkestad ST, Ulvik A, Ueland PM (2023) Assessment of vitamin B6 status in never-pregnant, pregnant and postpartum women and their infants. Eur J Nutr 62:867–878. https://doi.org/10.1007/s00394-022-03033-4
Acknowledgements
The authors wish to thank Ms. Sheryl Thomas-Nikpoor, Language Editor, Springer Publications, for her valuable comments in editing this manuscript.
Funding
The study was funded by the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences. This manuscript is extracted from the Ph.D. thesis of Maryam Mirani (Grant number: 23078).
Author information
Authors and Affiliations
Contributions
SMT and MHN participated in the study conception and design, data interpretation, and manuscript revision. MM, FM, ZD, and MD contributed to performing experimental methods and data acquiring and analyzing. MM and FM wrote the manuscript. SB advised some experimental protocols and was involved in the critical revision of the manuscript. All authors reviewed and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Medical Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1400.458) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirani, M., Bahmanpour, S., Masjedi, F. et al. Pyridoxamine protects human granulosa cells against advanced glycation end-products-induced steroidogenesis disturbances. Mol Biol Rep 50, 8537–8549 (2023). https://doi.org/10.1007/s11033-023-08723-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08723-8