Abstract
Background
Maxillary/mandibular bone marrow-derived mesenchymal stem cells (MBMSCs) exhibit a unique property of lower adipogenic potential than other bone marrow-derived MSCs. However, the molecular mechanisms regulating the adipogenesis of MBMSCs remain unclear. This study aimed to explore the roles of mitochondrial function and reactive oxygen species (ROS) in regulating the adipogenesis of MBMSCs.
Methods and results
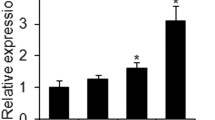
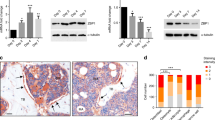
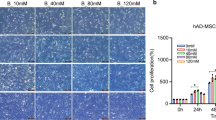
MBMSCs exhibited significantly lower lipid droplet formation than iliac BMSCs (IBMSCs). Moreover, the expression levels of CCAAT/enhancer-binding protein β (C/EBPβ), C/EBPδ, and early B cell factor 1 (Ebf-1), which are early adipogenic transcription factors, and those of peroxisome proliferator-activated receptor-γ (PPARγ) and C/EBPα, which are late adipogenic transcription factors, were downregulated in MBMSCs compared to those in IBMSCs. Adipogenic induction increased the mitochondrial membrane potential and mitochondrial biogenesis in MBMSCs and IBMSCs, with no significant difference between the two cell types; however, intracellular ROS production was significantly enhanced only in IBMSCs. Furthermore, NAD(P)H oxidase 4 (NOX4) expression was significantly lower in MBMSCs than in IBMSCs. Increased ROS production in MBMSCs by NOX4 overexpression or treatment with menadione promoted the expression of early adipogenic transcription factors but did not induce that of late adipogenic transcription factors or lipid droplet accumulation.
Conclusions
These results suggest that ROS may be partially involved in the process of MBMSC adipogenic differentiation from undifferentiated cells to immature adipocytes. This study provides important insights into the tissue-specific properties of MBMSCs.






Similar content being viewed by others
References
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. https://doi.org/10.1126/science.284.5411.143
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419. https://doi.org/10.1002/stem.1681
Yin JY, Luo XH, Feng WQ, Miao SH, Ning TT, Lei Q, Jiang T, Ma DD (2021) Multidifferentiation potential of dental-derived stem cells. World J Stem Cells 13:342–365. https://doi.org/10.4252/wjsc.v13.i5.342
Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG (2006) Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 38:758–768. https://doi.org/10.1016/j.bone.2005.10.027
Bugueno J, Li W, Salat P, Qin L, Akintoye SO (2017) The bone regenerative capacity of canine mesenchymal stem cells is regulated by site-specific multilineage differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol 123:163–172. https://doi.org/10.1016/j.oooo.2016.09.011
Onizuka S, Yamazaki Y, Park SJ, Sugimoto T, Sone Y, Sjoqvist S, Usui M, Takeda A, Nakai K, Nakashima K, Iwata T (2020) RNA-sequencing reveals positional memory of multipotent mesenchymal stromal cells from oral and maxillofacial tissue transcriptomes. BMC Genomics 21:417. https://doi.org/10.1186/s12864-020-06825-2
Miyata H, Ishii M, Suehiro F, Komabashiri N, Ikeda N, Sakurai T, Nishimura M (2023) Elucidation of adipogenic differentiation regulatory mechanism in human maxillary/mandibular bone marrow-derived stem cells. Arch Oral Biol 146:105608. https://doi.org/10.1016/j.archoralbio.2022.105608
Ambele MA, Dhanraj P, Giles R, Pepper MS (2020) Adipogenesis: a complex interplay of multiple molecular determinants and pathways. Int J Mol Sci 21:4283. https://doi.org/10.3390/ijms21124283
Hishida T, Nishizuka M, Osada S, Imagawa M (2009) The role of C/EBPdelta in the early stages of adipogenesis. Biochimie 91:654–657. https://doi.org/10.1016/j.biochi.2009.02.002
Almalki SG, Agrawal DK (2016) Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 92:41–51. https://doi.org/10.1016/j.diff.2016.02.005
Wu Z, Bucher NL, Farmer SR (1996) Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol 16:4128–4136. https://doi.org/10.1128/MCB.16.8.4128
Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED (2007) Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol 27:743–757. https://doi.org/10.1128/MCB.01557-06
Yan W, Diao S, Fan Z (2021) The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res Ther 12:140. https://doi.org/10.1186/s13287-021-02194-z
Hofmann AD, Beyer M, Krause-Buchholz U, Wobus M, Bornhauser M, Rodel G (2012) OXPHOS supercomplexes as a hallmark of the mitochondrial phenotype of adipogenic differentiated human MSCs. PLoS ONE 7:e35160. https://doi.org/10.1371/journal.pone.0035160
Zhang Y, Marsboom G, Toth PT, Rehman J (2013) Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 8:e77077. https://doi.org/10.1371/journal.pone.0077077
Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC (2013) Mitochondrial regulation in pluripotent stem cells. Cell Metab 18:325–332. https://doi.org/10.1016/j.cmet.2013.06.005
Zhang J, Xiang H, Liu J, Chen Y, He RR, Liu B (2020) Mitochondrial Sirtuin 3: new emerging biological function and therapeutic target. Theranostics 10:8315–8342. https://doi.org/10.7150/thno.45922
Kanda Y, Hinata T, Kang SW, Watanabe Y (2011) Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci 89:250–258. https://doi.org/10.1016/j.lfs.2011.06.007
Lee H, Lee YJ, Choi H, Ko EH, Kim JW (2009) Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284:10601–10609. https://doi.org/10.1074/jbc.M808742200
Suehiro F, Ishii M, Asahina I, Murata H, Nishimura M (2017) Low-serum culture with novel medium promotes maxillary/mandibular bone marrow stromal cell proliferation and osteogenic differentiation ability. Clin Oral Investig 21:2709–2719. https://doi.org/10.1007/s00784-017-2073-7
Greco SJ, Liu K, Rameshwar P (2007) Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells 25:3143–3154. https://doi.org/10.1634/stemcells.2007-0351
Funk MI, Conde MA, Piwien-Pilipuk G, Uranga RM (2021) Novel antiadipogenic effect of menadione in 3T3-L1 cells. Chem Biol Interact 343:109491. https://doi.org/10.1016/j.cbi.2021.109491
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y (2016) Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ 23:1128–1139. https://doi.org/10.1038/cdd.2015.168
Cao Z, Umek RM, McKnight SL (1991) Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5:1538–1552. https://doi.org/10.1101/gad.5.9.1538
Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA (1997) Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406–3410. https://doi.org/10.1074/jbc.272.6.3406
Atashi F, Modarressi A, Pepper MS (2015) The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev 24:1150–1163. https://doi.org/10.1089/scd.2014.0484
Schroder K, Wandzioch K, Helmcke I, Brandes RP (2009) Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29:239–245. https://doi.org/10.1161/ATVBAHA.108.174219
Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC, Liu GS (2013) Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22:878–888. https://doi.org/10.1089/scd.2012.0306
Chen X, Zhao C, Xu Y, Huang K, Wang Y, Wang X, Zhou X, Pang W, Yang G, Yu T (2021) Adipose-specific BMP and activin membrane-bound inhibitor (BAMBI) deletion promotes adipogenesis by accelerating ROS production. J Biol Chem 296:100037. https://doi.org/10.1074/jbc.RA120.014793
Hou Y, Xue P, Bai Y, Liu D, Woods CG, Yarborough K, Fu J, Zhang Q, Sun G, Collins S, Chan JY, Yamamoto M, Andersen ME, Pi J (2012) Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein beta during adipogenesis. Free Radic Biol Med 52:462–472. https://doi.org/10.1016/j.freeradbiomed.2011.10.453
Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N (1998) Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 47:1562–1569. https://doi.org/10.2337/diabetes.47.10.1562
Tirosh A, Potashnik R, Bashan N, Rudich A (1999) Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. A putative cellular mechanism for impaired protein kinase B activation and GLUT4 translocation. J Biol Chem 274:10595–10602. https://doi.org/10.1074/jbc.274.15.10595
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761. https://doi.org/10.1172/JCI21625
Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS (2011) Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14:537–544. https://doi.org/10.1016/j.cmet.2011.08.007
Yang C, Tibbitt MW, Basta L, Anseth KS (2014) Mechanical memory and dosing influence stem cell fate. Nat Mater 13:645–652. https://doi.org/10.1038/nmat3889
Acknowledgements
We thank Editage (www.editage.com) for English language proofing.
Funding
This work was supported in part by JSPS KAKENHI Grant Numbers JP20H03881 and JP21K10005.
Author information
Authors and Affiliations
Contributions
Conceptualization: MI; methodology: NI and MI; data analysis and interpretation: NI, MI, HM, YN, FS, NK, and TS; writing-original draft preparation: NI, MI, and MN; funding acquisition: MI and MN. All the authors have read and approved the submission of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Kagoshima University Hospital Clinical Research Committee, Kagoshima, Japan (no. 170263 EKI-KAI3).
Consent to participate
Written informed consent was obtained from all patients.
Consent to publish
The patients involved in this study provided informed consent for the publication of their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeda, N., Ishii, M., Miyata, H. et al. Role of reactive oxygen species (ROS) in the regulation of adipogenic differentiation of human maxillary/mandibular bone marrow-derived mesenchymal stem cells. Mol Biol Rep 50, 5733–5745 (2023). https://doi.org/10.1007/s11033-023-08528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08528-9