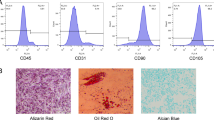
Abstract
Background
With an ageing population, the incidence of bone loss and obesity are increasing. Numerous studies emphasized the multidirectional differentiation ability of mesenchymal stem cells (MSCs), and reported betaine modulated the osteogenic differentiation and adipogenic differentiation of MSCs in vitro. We wondered how betaine affected the differentiation of hAD-MSCs and hUC-MSCs.
Methods and results
ALP staining and alizarin red S (ARS) staining were proved 10 mM betaine significantly increased the number of ALP-positive cells and plaque calcified extracellular matrices, accompanying by the up-regulation of OPN, Runx-2 and OCN. Oil red O staining demonstrated the number and size of lipid droplets were reduced, the expression of adipogenic master genes such as PPARγ, CEBPα and FASN were down-regulated simultaneously. For further investigating the mechanism of betaine on hAD-MSCs, RNA-seq was performed in none-differentiation medium. The Gene Ontology (GO) analysis showed fat cell differentiation and bone mineralization function terms were enriched, and KEGG showed PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction pathways were enriched in betaine treated hAD-MSCs, demonstrated betaine had a positive inducing effect on osteogenic of hAD-MSCs in the non-differentiation medium in vitro, which is opposite to the effect on adipogenic differentiation.
Conclusions
Our study demonstrated that betaine promoted osteogenic and compromised adipogenic differentiation of hUC-MSCs and hAD-MSCs upon low concentration administration. PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction were significantly enriched under betaine-treated. We showed hAD-MSCs were more sensitive to betaine stimulation and have a better differentiation ability than hUC-MSCs. Our results contributed to the exploration of betaine as an aiding agent for MSCs therapy.




Similar content being viewed by others
Data Availability
The datasets used and analyzed during the current study are available from the https://www.ncbi.nlm.nih.gov/sra/PRJNA849741, https://www.ncbi.nlm.nih.gov/sra/PRJNA885273.
Change history
12 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11033-023-08779-6
References
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
Kopelman PG (2000) Obesity as a medical problem. Nature 404(6778):635–643. https://doi.org/10.1038/35007508
Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R (2000) Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 28(6):707–715. https://doi.org/10.1016/s0301-472x(00)00160-0
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. https://doi.org/10.1186/1478-811X-9-12
Zhang C, Han X, Liu J, Chen L, Lei Y, Chen K et al (2022) Single-cell transcriptomic analysis reveals the Cellular heterogeneity of mesenchymal stem cells. https://doi.org/10.1016/j.gpb.2022.01.005. Genomics Proteomics Bioinformatics
Almalki SG, Agrawal DK (2016) Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 92(1–2):41–51. https://doi.org/10.1016/j.diff.2016.02.005
Liechty KW, Mackenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R et al (2000) Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6(11):1282–1286. https://doi.org/10.1038/81395
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147. https://doi.org/10.1126/science.284.5411.143
Schäffler A, Büchler C (2007) Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells 25(4):818–827. https://doi.org/10.1634/stemcells.2006-0589
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363(9419):1439–1441. https://doi.org/10.1016/S0140-6736(04)16104-7
Jaber H, Issa K, Eid A, Saleh FA (2021) The therapeutic effects of adipose-derived mesenchymal stem cells on obesity and its associated diseases in diet-induced obese mice. Sci Rep 11(1):6291. https://doi.org/10.1038/s41598-021-85917-9
Li L, Hui H, Jia X, Zhang J, Liu Y, Xu Q et al (2016) Infusion with human bone marrow-derived mesenchymal stem cells improves β-cell function in patients and non-obese mice with severe diabetes. Sci Rep 6:37894. https://doi.org/10.1038/srep37894
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI et al (2019) Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC Dosing is Superior to a single MSC dose and to Hyaluronic Acid in a controlled Randomized Phase I/II Trial. Stem Cells Transl Med 8(3):215–224. https://doi.org/10.1002/sctm.18-0053
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C et al (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14(8):493–507. https://doi.org/10.1038/s41581-018-0023-5
Takeuchi S, Tsuchiya A, Iwasawa T, Nojiri S, Watanabe T, Ogawa M et al (2021) Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen Med 6(1):19. https://doi.org/10.1038/s41536-021-00132-4
Kharbanda KK, Rogers DN, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC et al (2005) Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol 70(12):1883–1890. https://doi.org/10.1016/j.bcp.2005.09.021
Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ (2003) Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr 133(9):2845–2848. https://doi.org/10.1093/jn/133.9.2845
Yang Q, Yin W, Chen Y, Zhu D, Yin J, Zhang C et al (2019) Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed Pharmacother 120:109486. https://doi.org/10.1016/j.biopha.2019.109486
Villa I, Senesi P, Montesano A, Ferraretto A, Vacante F, Spinello A et al (2017) Betaine promotes cell differentiation of human osteoblasts in primary culture. J Transl Med 15(1):132. https://doi.org/10.1186/s12967-017-1233-5
Kornsuthisopon C, Nantanapiboon D, Rochanavibhata S, Nowwarote N, Namangkalakul W, Osathanon T (2022) Betaine promotes osteogenic differentiation in immortalized human dental pulp-derived cells. BDJ Open 8(1):31. https://doi.org/10.1038/s41405-022-00123-7
Joselit Y, Nanobashvili K, Jack-Roberts C, Greenwald E, Malysheva OV, Caudill MA et al (2018) Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr Diabetes 8(1):41. https://doi.org/10.1038/s41387-018-0035-z
Szkudelska K, Szkudelski T (2022) The anti-diabetic potential of betaine. Mechanisms of action in rodent models of type 2 diabetes. Biomed Pharmacother 150:112946. https://doi.org/10.1016/j.biopha.2022.112946
Chi Y, Han ZB, Xu FY, Wang YW, Feng XM, Chen F et al (2014) Adipogenic potentials of mesenchymal stem cells from human bone marrow, umbilical cord and adipose tissue are different. Zhongguo Shi Yan Xue Ye Xue Za Zhi 22(3):588–594. https://doi.org/10.7534/j.issn.1009-2137.2014.03.003
Hoshiba T, Kawazoe N, Chen G (2012) The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials 33(7):2025–2031. https://doi.org/10.1016/j.biomaterials.2011.11.061
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X et al (2009) A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18(4):545–559. https://doi.org/10.1089/scd.2008.0130
Yi X, Wu P, Liu J, Gong Y, Xu X, Li W (2019) Identification of the potential key genes for adipogenesis from human mesenchymal stem cells by RNA-Seq. J Cell Physiol 234(11):20217–20227. https://doi.org/10.1002/jcp.28621
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y et al (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114(12):1752–1761. https://doi.org/10.1172/JCI21625
Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ et al (1998) Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142(1):295–305. https://doi.org/10.1083/jcb.142.1.295
Page-Mccaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8(3):221–233. https://doi.org/10.1038/nrm2125
Chen J, Crawford R, Chen C, Xiao Y (2013) The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev 19(6):516–528. https://doi.org/10.1089/ten.TEB.2012.0672
Osyczka AM, Leboy PS (2005) Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology 146(8):3428–3437. https://doi.org/10.1210/en.2005-0303
Yu W, Chen Z, Zhang J, Zhang L, Ke H, Huang L et al (2008) Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol Cell Biochem 310(1–2):11–18. https://doi.org/10.1007/s11010-007-9661-9
Shaik S, Martin EC, Hayes DJ, Gimble JM, Devireddy RV (2019) Transcriptomic profiling of adipose derived stem cells undergoing osteogenesis by RNA-Seq. Sci Rep 9(1):11800. https://doi.org/10.1038/s41598-019-48089-1
Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC et al (2013) Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22(6):878–888. https://doi.org/10.1089/scd.2012.0306
Alshammari GM, Balakrishnan A (2019) Pumpkin (Cucurbita ficifolia Bouché) extract attenuate the adipogenesis in human mesenchymal stem cells by controlling adipogenic gene expression. Saudi J Biol Sci 26(4):744–751. https://doi.org/10.1016/j.sjbs.2018.10.002
Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E (2006) Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal 18(3):382–390. https://doi.org/10.1016/j.cellsig.2005.05.007
Dipilato LM, Ahmad F, Harms M, Seale P, Manganiello V, Birnbaum MJ (2015) The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Mol Cell Biol 35(16):2752–2760. https://doi.org/10.1128/MCB.00422-15
Carow B, Rottenberg ME (2014) SOCS3, a Major Regulator of infection and inflammation. Front Immunol 5:58. https://doi.org/10.3389/fimmu.2014.00058
Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM et al (2012) Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE 7(7):e39871. https://doi.org/10.1371/journal.pone.0039871
Biver E, Soubrier AS, Thouverey C, Cortet B, Broux O, Caverzasio J et al (2012) Fibroblast growth factor 2 inhibits up-regulation of bone morphogenic proteins and their receptors during osteoblastic differentiation of human mesenchymal stem cells. Biochem Biophys Res Commun 427(4):737–742. https://doi.org/10.1016/j.bbrc.2012.09.129
Funding
This work was supported by Liaoning Revitalization Talents Program (XLYC2002027), Programs from the Department of Education of Liaoning Province (LQ2020022). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
of competing interest.
Number of patent application: 202211292063 .X. The authors have no relevant financial or non-financial interests to disclose.
Author contributions Statement
Yue Jing and Jian Zhou designed and finished this study; Yue Jing analyze and visualize the data; Fenghua Guo validate the data; Lin Yu, Xiaomeng Ren and Xiushan Yin revised the paper. All authors reviewed and approved the final manuscript.
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Yue Jing and Jian Zhou contributed equally to this work.
The original online version of this article was revised: The missing equally contribution statement “The authors Yue Jing and Jian Zhou contributed equally to this work” is included.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, Y., Zhou, J., Guo, F. et al. Betaine regulates adipogenic and osteogenic differentiation of hAD-MSCs. Mol Biol Rep 50, 5081–5089 (2023). https://doi.org/10.1007/s11033-023-08404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08404-6