Abstract
Background
Due to the growing evidence of the importance of iron status in immune responses, the biomarkers of iron metabolism are of interest in novel Coronavirus Disease 2019 (COVID-19). The present prospective study was carried out to compare iron status indicated by levels of ferritin with the levels of two novel biomarkers related to iron homeostasis, hephaestin and hypoxia-inducible factors-1 (HIF-1α) in the serum of patients with COVID-19 in comparison with a control group.
Methods and results
Blood samples from 34 COVID-19 patients and from 43 healthy volunteers were collected and the levels of HEPH and HIF-1α were measured by ELISA and compared with levels of serum ferritin. COVID-19 patients had higher serum levels of ferritin than those levels in control group (P < 0.0001). Conversely levels of HIF-1α and HEPH in the COVID-19 group were significantly lower than those of control group (P < 0.0001 for both). An inverse correlation between hephaestin and ferritin as well as between HIF-1α and ferritin was found among all subjects (P < 0.0001), and among COVID-19 patients, but not to statistical significance.
Conclusion
Levels of hephaestin and HIF-1α were found to be inversely related levels of ferritin across all participants in the study, and to our knowledge this is the first report of hephaestin and HIF-1α as potential markers of iron status. Further studies are needed to corroborate the findings, utilizing a broader range of markers to monitor inflammatory as well as iron status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the report of World Health Organization (WHO), approximately two billion people in the world, more than 30% of the world population, have iron deficiency. Impaired iron metabolism results in alterations of the functionality of cells of the immune system. Anemia due to iron deficiency may cause the suppression of the immune system and decreased resistance to viral infections, including SARS-CoV-2 [1]. As the SARs-CoV-2 pandemic has unfolded numerous studies have collectively established the importance of a patient’s iron status in their response to COVID-19 [2]. A better understanding of biomarkers of iron status may help to identify factors that account for the wide heterogeneity of the clinical course and prognosis of COIVD-19 [2]. The iron-binding protein ferritin has been extensively studied in COVID-19 as an inflammatory biomarker, with marked hyperferritinemia being a nearly constant finding in severe disease [3]. In clinical practice, ferritin has been frequently included in routine evaluation of COVID-19 at hospital admission [4].
Ferritin is an iron-storage protein found in most cell types and is released into the serum from hepatocytes, macrophages and Kupffer cells and possibly other cell types [5]. Levels of serum ferritin are thought to reflect body iron stores such that low levels are a good indicator of iron deficiency anemia [5]. Inflammatory conditions can induce higher levels of serum ferritin which could prevent identification of a low body iron status. The dual roles of serum ferritin as a marker for both iron and status and inflammation can lead to ambiguity in interpretation, especially in the absence of an accurate measure of total body iron [6]. There is a need to explore other biomarkers as indicators of body iron status. Here we report the first analysis of serum hephaestin and HIF-1α as potential markers of iron status.
Hephaestin is a multi-copper ferroxidase (MCF) expressed in enterocytes that facilitates the absorption of dietary iron from the intestine [7,8,9] by facilitating export of iron through the basolateral membrane to the circulation. Iron is exported from enterocytes as Fe2+ via the basolateral transporter ferroportin and hephaestin converts Fe2+ to ferric Fe3+ for loading onto transferrin. It was discovered as the protein mutated in the sla locus of the sex-linked anemia (sla) mice. The microcytic anemia of the sla mouse was attributed to defective export of iron from enterocytes, indicating that decreased or defective hephaestin can lead to iron deficiency [7,8,9]. Infection of enterocytes with SARS-CoV-2 can induce down-regulation of ferroportin and hephaestin [10] and it was of interest to see if changes in serum hephaestin could correlate with COVID-19.
Hypoxia-inducible factors-1 (HIF-1) are cellular oxygen sensors that regulate metabolic changes due to hypoxia. The HIF-1 gene is activated by hypoxic conditions and the HIF-1 protein activates expression of genes involved in iron metabolism, angiogenesis and glucose metabolism [11]. The HIF-1 protein is unstable under normoxic conditions and HIF-1 stabilizers are a new class of drugs to treat anemia associated with chronic kidney disease, since they can reduce the effects of hypoxia and promote erythropoiesis [12]. It has been suggested that HIF-1 could have a protective role against COVID-19 [13], firstly by lowering the angiotensin-converting enzyme 2 (ACE2) levels and thereby limiting SARS-CoV-2 infection. Secondly, HIF-1 may be protective through its effects on promoting iron utilization and improving anemia [13]. Reduced levels of HIF-1 could be a factor that influences the outcome of SARS-CoV-2 infection.
The COVID-19 pandemic has led to a major worldwide health crisis [14], boosting an unprecedented expansion of research, in particular to understand the pathogenesis of the widely variable clinical manifestations of COVID-19, and the role of immune responses in these [15, 16]. Due to the growing evidence of the importance of iron status in immune responses, biomarkers of iron metabolism have become important in assessing COVID-19 severity and prognosis [2,3,4]. In the present study we compared levels of ferritin, an established marker, and two other proteins, hephaestin and HIF-1α as potential markers of iron metabolism, in the serum of patients with COVID-19 and to examine their response to SARS-CoV-2 infection.
Materials and methods
Selection criteria of patients
The study protocol was approved by the Ethical Committee of Non-interventional Clinical Research of Biruni University (Date: 30 Nov 2020, Number: 2020/45-31). Written informed consent was given by all the participants in the study, who were all volunteer patients. Also, none of the participants were minors, as patient ages are not included. All procedures were in accordance with the ethical standards of the Declaration of Helsinki.
Patients had applied to the emergency service and pandemic clinic of the Medical Faculty Research and Application Hospital of Sivas Cumhuriyet University with a COVID-19 pre-diagnosis between 1 June 2020-31 Dec 2020. A routine blood test was taken and a throat swab was taken for a PCR test for SARS-CoV-2. 34 patient samples whose diagnosis of COVID-19 was confirmed were randomly selected for this prospective study. Patients had been transferred to different hospitals so that the course and severity of their disease is not known. A control group was designed from 43 healthy volunteers who did not have any systemic disease and showed a similar distribution to the patient group in terms of sex and age and who donated venous blood samples for the tests.
Individuals with alcohol and substance abuse, those with acute or chronic diseases (including Diabetes Mellitus, hypertension, chronic kidney failure, heart failure, liver damage), those with autoimmune disorders and focus of infection, as well as those with unusual dietary habits (for instance eliminating certain food groups from the diet) were excluded from the study.
Collection and storage of samples
Approximately 3 mL of venous blood samples were collected from the patient and control groups, into hemogram tubes with EDTA and centrifuged for 5 min at 3000 rpm. The plasma were stored at − 20 °C until the tests were run. Then, the samples were brought to the room temperature and then the levels of hephaestin and HIF-1α were measured by Enzyme-linked immunosorbent test (ELISA) method as given below. The ferritin levels were collected from the routine blood testing from the patient record system.
Enzyme-linked immunosorbent test
To detect and quantify hephaestin and HIF-1α, ELISA protocols were applied according to the manufacturer’s instructions for the human hephaestin ELISA kit (AFG SCIENCE, EK712630) and human HIF-1 ELISA kit (AFG SCIENCE, EK710669). In brief, the diluted antibodies were added into wells of a 96-well ELISA plate. The plate was sealed to prevent evaporation and incubated for 15–18 h at 4 °C to immobilize the antibody. The diluted antibody was removed and the plate washed with washing solution. Blocking buffer was added to each well and incubated for 1 h at 37 °C to reduce non-specific binding of the target protein to the well. Blocking buffer was removed and the plate washed with the washing solution. Samples were diluted with sample dilution buffer and 100 µL of each sample was added to each well. For the calibration curve, a dilution series of the standard was prepared on the same plate. The plate was incubated for 1 h at 37 °C, samples and standards were removed and the plate washed with washing solution. The detection antibody was diluted in sample dilution buffer and 100 µL added to each well, then incubated for 1 h at 37 °C. After the reaction, the detection antibody was removed and the plate washed with the washing solution. Enzyme-labeled secondary antibody was diluted with sample dilution buffer and 100 µL was added to each well the incubated for 1 h at 37 °C. After the reaction, the secondary antibody was removed and the plate washed with washing solution. A substrate solution was added and allowed to incubate until the color developed. When the color has been developed sufficiently, a stop solution was added to stop the reaction. Then, the absorption was measured at 450 nm with a plate reader (Biotek Synergy HT Microplate Reader Multi-Mode).
Statistical analysis
The correct number of samples was determined by G*Power software (latest ver. 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany; http://www.gpower.hhu.de/). Considering a maximum difference of 0.20-unit increase in the variables and a standard deviation of 0.20, the power analysis gave an alpha value of 0.05, and the power of study as 95% if there would be at least 16 subjects in each group [17].
The data were tested by the Kolmogorov-Smirnov test for normality. Non-normally distributed variables of two groups were compared by a non-parametric Mann-Whitney test. All statistical analysis was performed by using GraphPad InStat program (Version 3.06, 2003).
Results
Of 34 patients with laboratory-confirmed SARS-CoV-2 infection, 21 patients were male (61.8%) and 13 were female (38.2%). The median age of patients was 50 years (Range 19–74). Of 43 healthy individuals in the control group, 8 were male (18.6%) and 35 were female (81.4%). The median age of these individuals was 45 years (Range 22–68).
The comparison of laboratory data showed that the COVID-19 patients had higher serum levels of ferritin than those levels in control group (P < 0.0001). However, the serum levels of HIF-1α and hephaestin in the COVID-19 group were significantly lower than those of control group (P < 0.0001 for both) (Table 1).
The correlation analysis of the laboratory data of all subjects indicated that there was a significant negative correlation between ferritin vs. hephaestin levels (P = 0.0007). However, this significance was not observed in separate groups (P > 0.05) (Table 2). The inverse correlation between hephaestin and ferritin levels was more pronounced in all subjects than those in each group (Fig. 1).
There was a significant positive correlation between HIF-1α vs. hephaestin levels for total of subjects (P < 0.0001). This significance was also observed in each group separately (P < 0.0001) (Table 2). The linear correlation between hephaestin and HIF-1α levels was pronounced in all subjects, healthy controls and COVID-19 patients (Fig. 2).
There was a significant negative correlation between ferritin vs. HIF-1α levels (P = 0.0016). However, this significance was not observed in separate groups (P > 0.05) (Table 2). The inverse correlation between HIF-1α and ferritin levels was more pronounced in all subjects than in each group (Fig. 3).
Discussion
Current understanding of the pathophysiology of COVID-19 suggests several pathways in which iron metabolism may be involved [1, 2, 18]. Viral infection could induce hypoxia via direct effects on the respiratory system, inducing an inflammatory response leading to anemia. Secondly, the bioavailability of iron could be reduced by activation of the innate immune system, which prevents the expansion of viral load in the acute-phase of the infection. This leads to the activation of hepcidin, an iron-regulating peptide hormone, which would increase retention of iron within cells such as macrophages or enterocytes, when normally the iron would be mobilised from these cells, primarily for erythropoiesis. The increased storage of iron leads to higher levels of ferritin and decreased erythropoiesis, resulting in hypoxia [2]. Finally, there have been reports that SARS-CoV-2 can suppress erythropoiesis by inducing an expansion of CD71 + erythroid cells (CECs) which have immunosuppressive properties [19, 20]. Expanded populations of CECs has been found to be negatively correlated with levels of hemoglobin in COVID-19 patients [20]. Erythroferrone is the iron regulator hormone which stimulates iron mobilization for erythropoiesis via modulation of hepcidin [21]. The lower levels of hemoglobin could be linked to lower levels of erythroferrone very recently reported in Covid 19 patients [22].
Elevated serum ferritin is considered a marker of inflammatory, autoimmune, infectious or malignant conditions [5, 23, 24], and has been found to vary according to the severity of COVID-19 as well as age, sex and presence of comorbidity among COVID-19 patients [25]. Consistent with these and other studies [22, 26,27,28,29] we found mean ferritin levels of COVID-19 patients to be about three times higher than in the control group (Table 1). Conversely the other two potential markers we measured were both significantly lower in Covid-19 patients compared with the control group, by 87% (hephaestin) and 70% (HIF-1α). We assessed whether there was an inverse correlation between ferritin and levels of hephaestin or HIF-1α and found that there was a significant correlation across all samples between ferritin and either hephaestin or HIF-1α, but not within patient or control groups (Table 2). These data suggest that measurement of serum hephaestin and HIF-1α may have potential use in assessing iron status.
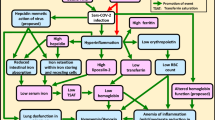
The observation that there are significantly decreased levels of hephaestin in COVID-19 patients would be consistent with hephaestin’s role in maintaining good iron status, which would be compromised in COVID-19 patients. In normal conditions, iron is taken up in the small intestine by divalent metal transporter-1 and is either stored in ferritin inside the mucosal cell or exported to the circulation by ferroportin. After iron is released from ferroportin it is oxidized by hephaestin and incorporated into transferrin (Fig. 4A). The origin of serum hephaestin has not been identified but is likely to be from enterocytes. The reduced levels of serum hephaestin in COVID-19 (Fig. 4B) could therefore reflect reduced activity in enterocytes and a reduced capacity for iron absorption across the gut.
A In normal conditions, Iron (ferritin-Fe2+) is converted to Fe3+ which is bound to transferrin to be delivered by other tissues. Fe2+ is continuously converted to Fe+3 by hephaestin. Therefore, Fe2+ level is decreasing while the expression of hephaestin is increasing. B In COVID-19, the hephaestin is inhibited. Ferritin (Fe+2) is accumulated within the cell and increased amount of Ferritin minimizes HIF-driven hypoxic response through its heavy chains by activating the asparaginyl hydroxylase. Thus, HIF expression is decreased
The importance of hephaestin is considered in extra-intestinal tissues for maintaining whole-body iron metabolism, and the lack of HEPH is suggested to result in increased non-transferrin bound iron.
Levels of HIF-1α were also significantly lower in COVID-19 patients compared with the control group.
During the condition of hypoxemia which can occur in COVID-19 infection, the angiotensin converting enzyme 1 (ACE-1) is upregulated by HIF-1 while the expression of ACE-2 is markedly decreased [30]. It has been suggested that induced expression of ACE-2 is positively associated with COVID-19 infection [25]. Thus, both hypoxemia and related ACE-2 upregulation may reflect lower levels of HIF-1 expression after infection with SARS-CoV-2.
In the present study, the serum levels of HIF-1α in COVID-19 patients were directly proportional with the levels of hephaestin but inversely proportional with the levels of ferritin, although not at statistical significance (Table 2). Changes in HIF-1, associated with reduction in ACE-2 levels and hypoxia may be predictive factors for the presence of the disease. Ultimately, an altered iron metabolism response detected by elevated levels of ferritin and reduced levels of hephaestin and or HIF-1α in SARS-CoV-2 infection may be valuable for risk stratification and for treatment options of COVID-19. In COVID-19 cases, the increased levels of ferritin in severe disease might indicate an underlying dysregulated iron metabolism response against the infection (Fig. 4). Whether the ferritin combined with measurement of hephaestin and HIF-1α can be used for prognostic purposes, or have further implications for identifying novel treatment targets, needs further investigation.
The relatively small numbers of patients is a limitation of the study and the analysis needs to be repeated with larger numbers across centers to confirm the correlation. Moreover, it would be very helpful to carry out a longitudinal study to know the severity of the disease that developed in each patient and to know how the disease was resolved. This was not possible in the current study and is another limitation. The cross-section design of the study also precludes assessing causality. The differences in sex ratio in the control and Covid-19 positive groups could also be a confounding factor [19], with a high proportion of males in the Covid group and a high proportion of females in the control group, although in some studies differences in ferritin levels between sexes was not assessed [22, 27] or not found [28]. Finally, a further limitation of the study was the lack data available for serum iron levels and associated parameters levels of transferrin and % saturation of serum transferrin. These measurements would provide a more definitive assessment of iron status [22], which should be addressed in future studies.
In conclusion, this report presents, for the first time, measurements of serum hephaestin and HIF-1a in COVID-19 patients, in which levels of both markers are significantly lower in COVID-19 patients. In common with many other studies levels of serum ferritin were significantly higher in COVID-19 patients. It remains to be seen if and how the two novel markers are linked to a patient’s iron status, but the results suggest further investigation of these markers may be useful.
Data availability
Data available on request from the authors.
References
Girelli D, Marchi G, Busti F, Vianello A (2021) Iron metabolism in infections: focus on COVID-19. Semin Hematol 58(3):182–187. https://doi.org/10.1053/j.seminhematol.2021.07.001
Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa-Díaz ZM, Salvador D Jr, Groothof D, Minder B, Kopp-Heim D, Hautz WE, Eisenga MF, Franco OH, Glisic M, Muka T (2020) Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol 35(8):763–773. doi: https://doi.org/10.1007/s10654-020-00678-5
Cheng L, Li H, Li L, Liu C (2020) Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal 34(10):e23618
Chen Z, Xu W, Ma W (2021) Clinical laboratory evaluation of COVID-19. Clin Chim Acta 519:172–182
Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV (2010) Serum ferritin: past, present and future. Biochim Biophys Acta 1800(8):760–769. doi: https://doi.org/10.1016/j.bbagen.2010.03.011
DePalma RG, Hayes VW, O’Leary TJ (2021) Optimal serum ferritin level range: iron status measure and inflammatory biomarker. Metallomics 13(6):mfab030. doi: https://doi.org/10.1093/mtomcs/mfab030
Vulpe Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ (1999) Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21(2):195–199. doi: https://doi.org/10.1038/5979
Fuqua BK, Lu Y, Frazer DM, Darshan D, Wilkins SJ, Dunn L, Loguinov AV, Kogan SC, Matak P, Chen H, Dunaief JL, Vulpe CD, Anderson GJ (2018) Severe iron metabolism defects in mice with double knockout of the multicopper ferroxidases hephaestin and ceruloplasmin. Cell Mol Gastroenterol Hepatol 6(4):405–427
Aslan ES, White KN, Syed BA, Srai KS, Evans RW (2020) Expression of soluble, active, fluorescently tagged hephaestin in COS and CHO cell lines. Turkish J Biology 44(6):393–405
Ward JL, Torres-Gonzalez M, Ammons MCB (2022) The influence of viral infections on iron homeostasis and the potential for lactoferrin as a therapeutic in the age of the SARS-CoV-2 pandemic. Nutrients 14(15):3090
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70(5):1469–1480. doi: https://doi.org/10.1124/mol.106.027029
Serebrovska ZO, Chong EY, Serebrovska TV, Tumanovska LV, Xi L, Hypoxia (2020) HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin 41(12):1539–1546. doi: https://doi.org/10.1038/s41401-020-00554-8
Afsar B, Kanbay M, Afsar RE (2020) Hypoxia inducible factor-1 protects against COVID-19: a hypothesis. Med Hypotheses 143:109857. doi:https://doi.org/10.1016/j.mehy.2020.109857
Blumenthal D, Fowler EJ, Abrams M, Collins SR (2020) COVID-19—implications for the health care system. N Engl J Med 383(15):1483–1488
Else H (2020) How a torrent of COVID science changed research publishing—in seven charts. Nature 588(7839):553
Brodin P (2021) Immune determinants of COVID-19 disease presentation and severity. Nat Med 27(1):28–33
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioural and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/bf03193146
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. https://doi.org/10.1016/j.jaut.2020.102433
Elahi S (2022) Hematopoietic responses to SARS-CoV-2 infection. Cell Mol Life Sci 79(3):187. https://doi.org/10.1007/s00018-022-04220-6
Shahbaz S, Xu L, Osman M, Sligl W, Shields J, Joyce M, Tyrrell DL, Oyegbami O, Elahi S (2021) Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2. Stem Cell Rep 16(5):1165–1181. https://doi.org/10.1016/j.stemcr.2021.04.001
Kautz L, Jung G, Nemeth E, Ganz T (2014) Erythroferrone contributes to recovery from anemia of inflammation. Blood 124(16):2569–74. https://doi.org/10.1182/blood-2014-06-584607
Delaye JB, Alarcan H, Vallet N, Veyrat-Durebex C, Bernard L, Hérault O, Ropert M, Marlet J, Gyan E, Andres C, Blasco H, Piver E (2022) Specific changes of erythroid regulators and hepcidin in patients infected by SARS-COV-2. J Investig Med 70(4):934–938. https://doi.org/10.1136/jim-2021-002270
Mahroum N, Alghory A, Kiyak Z, Alwani A, Seida R, Alrais M, Shoenfeld Y (2022) Ferritin—from iron, through inflammation and autoimmunity, to COVID-19. J Autoimmun 126:102778. https://doi.org/10.1016/j.jaut.2021.102778
Habib HM, Ibrahim S, Zaim A, Ibrahim WH (2021) The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother 136:111228. https://doi.org/10.1016/j.biopha.2021.111228
Kaushal K, Kaur H, Sarma P, Bhattacharyya A, Sharma DJ, Prajapat M, Pathak M, Kothari A, Kumar S, Rana S, Kaur M, Prakash A, Mirza AA, Panda PK, Vivekanandan S, Omar BJ, Medhi B, Naithani M (2022) Serum ferritin as a predictive marker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care 67:172–181. https://doi.org/10.1016/j.jcrc.2021.09.023
Tural Onur S, Altın S, Sokucu SN, Fikri B, Barça T, Bolat E, Toptaş M (2021) Could ferritin level be an indicator of COVID-19 disease mortality? J Med Virol 93(3):1672–1677. doi: https://doi.org/10.1002/jmv.26543
Dahan S, Segal G, Katz I, Hellou T, Tietel M, Bryk G, Amital H, Shoenfeld Y, Dagan A (2020) Ferritin as a marker of severity in COVID-19 patients: a fatal correlation. Isr Med Assoc J 22(8):494–500
Hegelund MH, Glenthøj A, Ryrsø CK, Ritz C, Dungu AM, Sejdic A, List KCK, Krogh-Madsen R, Lindegaard B, Kurtzhals JAL, Faurholt-Jepsen D (2022) Biomarkers for iron metabolism among patients hospitalized with community-acquired pneumonia caused by infection with SARS-CoV-2, bacteria, and influenza. APMIS 130(9):590–596. https://doi.org/10.1111/apm.13259
Bastin A, Shiri H, Zanganeh S, Fooladi S, Momeni Moghaddam MA, Mehrabani M, Nematollahi MH (2021) Iron chelator or iron supplement consumption in COVID-19? The role of iron with severity infection. Biol Trace Elem Res 25:1–11. https://doi.org/10.1007/s12011-021-03048-8
Zhang R, Wu Y, Zhao M (2009) Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 297:L631–L640
Acknowledgements
Thanks for all authors and Biruni University for the grand support of project: Biruni-BAP-2020-01-09.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Ethical approval
The study protocol was approved by the Ethical Committee of Non-interventional Clinical Research of BiruniUniversity (Date: 30 Nov 2020, Number: 2020/45-31). All procedures were in accordance with the ethical standards of the Declaration of Helsinki.
Informed consent
A written informed consent was given by all the participants in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslan, E.S., Aydın, H., Tekin, Y.K. et al. Association between iron metabolism and SARS-COV-2 infection, determined by ferritin, hephaestin and hypoxia-induced factor-1 alpha levels in COVID-19 patients. Mol Biol Rep 50, 2471–2478 (2023). https://doi.org/10.1007/s11033-022-08221-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08221-3