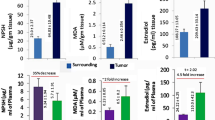
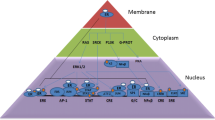
Abstract
Human estrogen sulfotransferase (SULT1E1) and nuclear factor erythroid 2-related factor 2 (Nrf-2) expression influences each other in advanced human breast carcinogenesis. The difference in the metabolism of estradiol (E2) in pre- and post-menopausal women remains to be connected with post-menopausal breast cancer. A synergism between ROS production and E2 generation has been demonstrated. No definite mechanism for simultaneous functions of Nrf2, oxidative stress E2 regulating enzymes (SULT1E1) has been yet clarified. Our present review demonstrates that ROS dependent regulation of Nrf-2 is one of the most important determinants of E2 regulation by altering SULT1E1 expression. This study also focuses the idea that estrogen receptor cased subtypes of cancer may have different molecular environments which has an impact on the therapeutic efficacy.



Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study available on request.
Abbreviations
- SULT1E1:
-
Estrogen sulfotransferase
- Nrf-2:
-
Nuclear factor erythroid 2-related factor 2
- VAT:
-
Visceral adipose tissue
- 16αOHE1:
-
16α-Hydroxyestrone
- STa:
-
Hydroxysteroid sulfotransferase
- MTX:
-
Methotrexate
- AST-IV:
-
Aryl sulfotransferase IV
- MDA:
-
Malondialdehyde
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate hydrogen
- MDA DG:
-
Deoxyguanosine malondialdehyde
- MDA DA:
-
Deoxyadenosine malondialdehyde
- ENU:
-
Ethyl nitroso urea
- ARE:
-
Antioxidant response element
- HO-1:
-
Heme oxygenase-1
- GPX:
-
Glutathione peroxidase 1
- NQO1:
-
NADPH dehydrogenase, quinone 1
- GST:
-
Glutathionine S transferase
- SOD:
-
Superoxide dismutase
- Keap1:
-
Kelch-like ECH-associated protein 1
- DLG:
-
Low-affinity binding site
- LPS:
-
Lipopolysaccharides
- CBP:
-
Cyclic-AMP response element binding protein
- MafF:
-
Musculoaponeurotic fibrosarcoma homolog F
- MafG:
-
Musculoaponeurotic fibrosarcoma homolog G
- MafK:
-
Musculoaponeurotic fibrosarcoma homolog K
- HNF4α:
-
Hepatocyte nuclear factor 4α
- FXR:
-
Farnesoid X receptor
- PXR:
-
Pregnane X receptor
References
Motohashi H, Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10:549–557
Klaassen CD, Slitt AL (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328
Mandlekar S, Hong JL, Kong AN (2006) Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metab 7:661–675
Fares MY, Salhab HA, Khachfe HH, Khachfe HM (2019) Breast cancer epidemiology among Lebanese women: an 11-year analysis. Medicina (Kaunas) 10(55):8
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM (2017) Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 67(5):378–397
Sowers M, McConnell D, Jannausch ML, Randolph JF, Brook R, Gold EB, Crawford S, Lasley B (2008) Oestrogen metabolites in relation to isoprostanes as a measure of oxidative stress. Clin Endocrinol (Oxf) 68(5):806–813
Xu Y, Liu X, Guo F, Ning Y, Zhi X, Wang X, Chen S, Yin L, Li X (2012) Effect of estrogen sulfation by SULT1E1 and PAPSS on the development of estrogen-dependent cancers. Cancer Sci 103:1000–1009
Maiti S, Nazmeen A (2019) Impaired redox regulation of estrogen metabolizing proteins is important determinant of human breast cancer. Cancer Cell Int 19:111
Guo Y, Hu B, Huang H, Tsung A, Gaikwad NW, Xu M, Jiang M, Ren S, Fan J, Billiar TR, Huang M, Xie W (2015) Estrogen sulfotransferase is an oxidative stress-responsive gene that gender-specifically affects liver ischemia/reperfusion injury. J Biol Chem 290(23):14754–14764
Chen G, Yin S, Maiti S, Shao X (2002) 4-Hydroxytamoxifen sulfation metabolism. J Biochem Mol Toxicol 16(6):279–285
Maiti S, Chen G (2003) Methotrexate is a novel inducer of rat liver and intestinal sulfotransferases. Arch Biochem Biophys 418(2):161–168
Maiti S, Chen G (2015) Ethanol up-regulates phenol sulfotransferase (SULT1A1) and hydroxysteroid sulfotransferase (SULT2A1) in rat liver and intestine. Arch Physiol Biochem 121(2):68–74
Maiti S, Chen X, Chen G (2005) All-trans retinoic acid induction of sulfotransferases. Basic Clin Pharmacol Toxicol 96(1):44–53
Sajadimajd S, Khazaei M (2018) Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets 18(6):538–557
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950
Brown NS, Bicknell R (2001) Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3(5):323–327
Nazmeen A, Chen G, Ghosh TK, Maiti S (2020) Breast cancer pathogenesis is linked to the intra-tumoral estrogen sulfotransferase (hSULT1E1) expressions regulated by cellular redox dependent Nrf-2/NFκβ interplay. Cancer Cell Int 4(20):70
Wang M, Dhingra K, Hittelman WN, Liehr JG, de Andrade M, Li D (1996) Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol Biomark Prev 5(9):705–710
Vander Veen LA, Hashim MF, Shyr Y, Marnett LJ (2003) Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc Natl Acad Sci USA 100(24):14247–14252
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462
Lena S, Svoboda M, Klameth L et al (2013) The sulfatase pathway for estrogen formation: targets for the treatment and diagnosis of hormone-associated tumors. J Drug Deliv 95:7605
Yali Xu, Lin X, Jiawen Xu, Jing H, Qin Y, Li Y (2018) SULT1E1 inhibits cell proliferation and invasion by activating PPARγ in breast cancer. J Cancer 9(6):1078–1087
Maiti S, Zhang J, Chen G (2007) Redox regulation of human estrogen sulfotransferase (hSULT1E1). Biochem Pharmacol 73(9):1474–1481
Nazmeen A, Maiti S (2018) Oxidant stress induction and signalling in xenografted (human breast cancer-tissues) plus estradiol treated or N-ethyl-N-nitrosourea treated female rats via altered estrogen sulfotransferase (rSULT1E1) expressions and SOD1/catalase regulations. Mol Biol Rep 45(6):2571–2584
Li W, Yu S, Liu T, Kim JH, Blank V, Li H, Kong AN (2008) Heterodimerization with small Maf proteins enhances nuclear retention of Nrf2 via masking the NESzip motif. Biochim Biophys Acta 1783(10):1847–1856
Li W (2009) Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48(2):91–104
Liu WJ, Ye L, Huang WF (2016) p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett 21:29
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Puissant A, Fenouille N, Auberger P (2012) When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res 2:397–413
Zheng Q, Su H, Ranek MJ, Wang X (2011) Autophagy and p62 in cardiac proteinopathy. Circ Res 109:296–330
Jixiang Z, Wang X, Vikash V (2016) ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 435:965
Petrovski G, Zahuczky G, Katona K (2007) Clearance of dying autophagic cells of different origin by professional and non-professional phagocytes. Cell Death Differ 14:1117–1128
Totta P, Busonero C, Leone S (2016) Dynamin II is required for 17β-estradiol signaling and autophagy-based ERα degradation. Sci Res 6:23727
Lahm T, Petrache I (2012) LC3 as a potential therapeutic target in hypoxia-induced pulmonary hypertension. Autophagy 8:1146–1147
Guido C, Panza S, Santoro M (2012) Estrogen receptor beta (ERβ) produces autophagy and necroptosis in human seminoma cell line through the binding of the Sp1 on the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) promoter gene. Cell Cycle 11:2911–2921
Hsieh DJ, Kuo W-W, Lai Y-P (2015) 17β-Estradiol and/or estrogen receptor β attenuate the autophagic and apoptotic effects induced by prolonged hypoxia through HIF-1α-mediated BNIP3 and IGFBP-3 signaling blockage. Cell Physiol Biochem 36:274–284
Yang Y, Zheng X, Li B (2014) Increased activity of osteocyte autophagy in ovariectomized rats and its correlation with oxidative stress status and bone loss. Biochem Biophys Res Commun 451:86–92
Li W, Siwang Yu, Liu T, Kim J-H, Blank V, Hong Li A-N, Kong T (2008) Heterodimerization with small Maf proteins enhances nuclear retention of Nrf2 via masking the NESzip Motif. Biochim Biophys Acta 1783(10):1847–1856
Hung HL, Kim AY, Hong W, Rakowski C, Blobel GA (2001) Stimulation of NF-E2 DNA binding by CREB-binding protein (CBP)-mediated acetylation. J Biol Chem 276(14):10715–10721
Bensellam M, Montgomery MK, Luzuriaga J, Chan JY, Laybutt DR (2015) Inhibitor of differentiation proteins protect against oxidative stress by regulating the antioxidant-mitochondrial response in mouse beta cells. Diabetologia 58(4):758–770
Liu X, Xue R, Yang C, Gu J, Chen S, Zhang S (2018) Cholestasis-induced bile acid elevates estrogen level via farnesoid X receptor-mediated suppression of the estrogen sulfotransferase SULT1E1. J Biol Chem 293(33):12759–12769
Hong Lu, Gonzalez FJ, Klaassen C (2010) Alterations in hepatic mRNA expression of phase II enzymes and xenobiotic transporters after targeted disruption of hepatocyte nuclear factor 4 alpha. Toxicol Sci 118(2):380–390
Acknowledgements
University Grants Commission, New Delhi provided JRF and SRF to AN who is a Ph.D. students working in the Post Graduate Department of Biochemistry, OIST.
Funding
Institutional, no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazmeen, A., Chen, G. & Maiti, S. Dependence between estrogen sulfotransferase (SULT1E1) and nuclear transcription factor Nrf-2 regulations via oxidative stress in breast cancer. Mol Biol Rep 47, 4691–4698 (2020). https://doi.org/10.1007/s11033-020-05518-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05518-z