Abstract
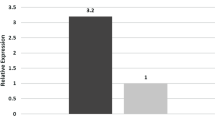
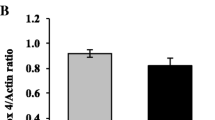
To evaluate changes in the inflammatory response of thioredoxin (TXN), thioredoxin interacting protein (TXNIP), transducer and activator of transcription 3, NFƙB-p50 and STAT3 at the level of maternal serum, placenta, and umbilical cord blood of women with gestational diabetes mellitus type 2 (GDMA2) compared to normal pregnancies (NP). Thirty pregnant women (20 with GDMA2 and 10 NP) were recruited during admission for delivery. Blood samples were obtained from the parturients and umbilical cords, as well as placental tissue for mRNA and protein extraction. TXNIP mRNA expression was significantly increased in maternal serum of women with GDMA2 compared to NP women. TXNIP mRNA was significantly decreased in GDMA2 placentas and cord blood compared to NP. TXN/TXNIP mRNA ratio showed significantly high absolute values in placental and cord blood (2.39 and 1.66) respectively, compared to maternal ratio (1.084) (P < 0.001). TXN/TXNIP placenta protein ratio showed similar values between GDMA2 and NP (0.98 and 0.86; P = 0.7). STAT3 and its target protein SOCS3, as well as NFƙB-p50 mRNA expression were significantly increased in placentas of GDMA2. NFƙB-p50 mRNA expression was significantly decreased in cord blood compared to both maternal and placental mRNA expression. Pro-inflammatory changes are expressed by low mRNA TXN/TXNIP ratio in maternal blood of GDMA2 patients, but not in placental and umbilical cord blood samples. This, as well as the feedback role of SOCS3 in STAT3 pathway and NFƙB-p50 expression, may indicate that the placenta has a role in protecting the fetus from damage due to inflammatory response, which is common in diabetes.



Similar content being viewed by others
Abbreviations
- GDM:
-
Gestational diabetes mellitus
- GDMA2:
-
Gestational Diabetes Mellitus class A2
- TXNIP:
-
Thioredoxin interacting protein
- TXN:
-
Thioredoxin
References
Hod M, Pretty M, Mahmood T, FIGO, EAPM and EBCOG (2018) Joint position statement on universal screening for GDM in Europe by FIGO, EBCOG and EAPM. Eur J Obstet Gynecol Reprod Biol 228:329–330
American Diabetes A (2018) Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care 41:S137–S143
Pasternak Y, Aviram A, Poraz I, Hod M (2013) Maternal nutrition and offspring's adulthood NCD's: a review. J Mater Fetal Neonatal Med 26:439–444
Collet JF, Messens J (2010) Structure, function, and mechanism of thioredoxin proteins. Antioxid Redox Signal 13:1205–1216
Arner ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267:6102–6109
Nakatsukasa Y, Tsukahara H, Tabuchi K et al (2013) Thioredoxin-1 and oxidative stress status in pregnant women at early third trimester of pregnancy: relation to maternal and neonatal characteristics. J Clin Biochem Nutr 52:27–31
Chong CR, Chan WP, Nguyen TH et al (2014) Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther 28:347–360
Corbett JA (2008) Thioredoxin-interacting protein is killing my beta-cells! Diabetes 57:797–798
Shalev A (2014) Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Mol Endocrinol 28:1211–1220
Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A (2014) Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J Biol Chem 289:11807–11815
Oka S, Yoshihara E, Bizen-Abe A et al (2009) Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxisome proliferator-activated receptor function. Endocrinology 150:1225–1234
Parikh H, Carlsson E, Chutkow WA et al (2007) TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4:e158
Li M, Zhang Y, Cao Y et al (2018) Icariin ameliorates palmitate-induced insulin resistance through reducing thioredoxin-interacting protein (TXNIP) and suppressing ER stress in C2C12 myotubes. Front Pharmacol 9:1180
Harder-Lauridsen NM, Krogh-Madsen R, Holst JJ et al (2014) Effect of IL-6 on the insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 306:E769–E778
Galoczova M, Coates P, Vojtesek B (2018) STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett 23:12
Inoue H, Ogawa W, Ozaki M et al (2004) Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 10:168–174
Emanuelli B, Peraldi P, Filloux C et al (2001) SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 276:47944–47949
Shi H, Tzameli I, Bjorbaek C, Flier JS (2004) Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem 279:34733–34740
Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G (2012) The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol 22:557–566
Baker RG, Hayden MS, Ghosh S (2011) NF-kappaB, inflammation, and metabolic disease. Cell Metab 13:11–22
Coustan DR, Carpenter MW (1998) The diagnosis of gestational diabetes. Diabetes Care 21(Suppl 2):B5–B8
American Diabetes A (2019) Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 42:S13–S28
Zitman-Gal T, Einbinder Y, Ohana M, Katzav A, Kartawy A, Benchetrit S (2019) Effect of liraglutide on the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway in diabetic kidney disease in db/db mice and in cultured endothelial cells. J Diabetes 11:656–664
Zitman-Gal T, Green J, Pasmanik-Chor M, Oron-Karni V, Bernheim J (2010) Endothelial pro-atherosclerotic response to extracellular diabetic-like environment: possible role of thioredoxin-interacting protein. Nephrol Dial Transplant 25:2141–2149
Zitman-Gal T, Golan E, Green J, Bernheim J, Benchetrit S (2012) Vitamin D receptor activation in a diabetic-like environment: potential role in the activity of the endothelial pro-inflammatory and thioredoxin pathways. J Steroid Biochem Mol Biol 132:1–7
Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT (2006) The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem 281:21884–21891
Chutkow WA, Patwari P, Yoshioka J, Lee RT (2008) Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J Biol Chem 283:2397–2406
Clausen TD, Mathiesen ER, Hansen T et al (2008) High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31:340–346
Clausen TD, Mathiesen ER, Hansen T et al (2009) Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 94:2464–2470
Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT (2004) Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 279:30369–30374
Eren E, Aykal G, Sayrac S, Erol O, Ellidag HY, Yilmaz N (2017) Relationship between thioredoxin and thioredoxin-binding protein in patients with gestational diabetes mellitus. J Matern Fetal Neonatal Med 30:164–168
Liu T, Zhang L, Joo D, Sun SC (2017) NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2:17023
Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP (2009) Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol 221:262–272
Correa-Silva S, Alencar AP, Moreli JB et al (2018) Hyperglycemia induces inflammatory mediators in the human chorionic villous. Cytokine 111:41–48
Sato BL, Sugawara A, Ward MA, Collier AC (2014) Single blastomere removal from murine embryos is associated with activation of matrix metalloproteinases and Janus kinase/signal transducers and activators of transcription pathways of placental inflammation. Mol Hum Reprod 20:1247–1257
Goossens V, Harton G, Moutou C, Traeger-Synodinos J, Van Rij M, Harper JC (2009) ESHRE PGD consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod 24:1786–1810
Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR (2008) Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update 14:335–344
Zhou X, Jiang Z, Zou Y, Yin Y, Zuo Q, Sun L (2015) Role of SOCS3 in the Jak/stat3 pathway in the human placenta: different mechanisms for preterm and term labor. Acta Obstet Gynecol Scand 94:1112–1117
Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N et al (2012) The expression of suppressor of cytokine signaling 1 and 3 in fat and placental tissue from women with gestational diabetes. Gynecol Endocrinol 28:841–844
Xu G, Chen J, Jing G, Shalev A (2013) Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med 19:1141–1146
Hu Q, Wei B, Wei L et al (2015) Sodium tanshinone IIA sulfonate ameliorates ischemia-induced myocardial inflammation and lipid accumulation in Beagle dogs through NLRP3 inflammasome. Int J Cardiol 196:183–192
Acknowledgements
We thank the delivery room staff of Meir Medical Center for helping recruit women to the study. We thank Faye Schreiber for editing the manuscript and Navah Jelin for assistance with the statistical analysis. They are both employees of Meir Medical Center.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.P; T.B.S; K.C.H;S.B and T.Z.G conception and design of research; Y.P; M.O and T.Z.G data and blood collection; Y.P; M.O and T.Z.G performed experiments; Y.P ;M.O; K.C.H and T.Z.G analyzed data, interpreted results and prepared figures; Y.P, T.B.S; K.C.H; S.B and T.Z.G drafted manuscript; Y.P ; T.B.S ;S.B and T.Z.G edited and revised manuscript; all authors approved the final version of manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Meir Medical Center local Ethics Committee (approval no. 0132-16-MMC)
Informed consent
Informed consent was obtained from all individual participants included in the study prior to enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pasternak, Y., Ohana, M., Biron-Shental, T. et al. Thioredoxin, thioredoxin interacting protein and transducer and activator of transcription 3 in gestational diabetes. Mol Biol Rep 47, 1199–1206 (2020). https://doi.org/10.1007/s11033-019-05221-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05221-8