Abstract
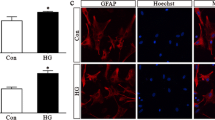
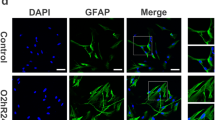
Oligodendrocyte precursor cells (OPC) are a uniformly distributed population of glial cells that are well known for proliferating and differentiating into mature oligodendrocytes to form the myelin sheet in the central nervous system (CNS). Since monocarboxylate transporter 1 (MCT1) has shown to be expressed by oligodendroglia, the involvement of these cells with the metabolic support to axons has emerged as an important role in the maintenance of neuronal functionality. Hyperglycemia is a metabolic dysfunction highly associated with oxidative stress, a classical feature linked to many disorders such as diabetes mellitus. Despite of being widely investigated in several different cell cultures, including astrocytes and neurons, such condition has been poorly investigated in OPC culture. Thus, the aim of this study was to explore the possible effects of high-glucose exposure in acute and chronic conditions on oligodendroglial development and functionality in vitro. In this sense, we have demonstrated that under high-glucose exposure OPC improved its differentiation rate without affecting its membrane integrity and its morphology. Besides, chronic high-glucose condition also increased glucose uptake and lactate release. On the other hand, our findings also showed that, unlike what happens in other glial cells and neurons, high-glucose exposure did not seem to induce oxidative stress in OPC culture. Therefore, as far as we have investigated in this present study, we suggest that OPC may be able to support neurons and other glial cells during hyperglycemia events.





Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- DCFH-DA:
-
2′-7′-Dichlorofluorescein diacetate
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- FBS:
-
Fetal bovine serum
- GSH:
-
Glutathione
- HBSS:
-
Hank’s balanced salt solution
- HPLCH:
-
High performance liquid chromatography
- MCT1:
-
Monocarboxylate transporter 1
- NG2:
-
Neuron–glial antigen 2
- OPC:
-
Oligodendrocyte precursor cells
- PI:
-
Propidium iodide
- ROS:
-
Reactive oxygen species
References
Aggarwal S, Yurlova L, Simons M (2011) Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol 21:585–593
Bahniwal M, Little JP, Klegeris A (2017) High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res 14:731–741. https://doi.org/10.2174/1567205014666170117104053
Bankston AN, Mandler MD, Feng Y (2013) Oligodendroglia and neurotrophic factors in neurodegeneration. Neurosci Bull 29:216–228. https://doi.org/10.1007/s12264-013-1321-3
Barateiro A, Fernandes A (2014) Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta 1843:1917–1929
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Bergersen LH (2007) Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 145:11–19
Biswas SK (2016) Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev 2016:5698931. https://doi.org/10.1155/2016/5698931
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. https://doi.org/10.1385/0-89603-472-0:347
Cater HL, Chandratheva A, Benham CD, Morrison B, Sundstrom LE (2003) Lactate and glucose as energy substrates during, and after, oxygen deprivation in rat hippocampal acute and cultured slices. J Neurochem 87:1381–1390
Chamberlain KA, Nanescu SE, Psachoulia K, Huang JK (2016) Oligodendrocyte regeneration: its significance in myelin replacement and neuroprotection in multiple sclerosis. Neuropharmacology 110:633–643
Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476–488
Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J (2004) Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci 27:453–465
Domingues HS, Cruz A, Chan JR, Relvas JB, Rubinstein B, Pinto IM (2018) Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia 66:5–14. https://doi.org/10.1002/glia.23206
Ettle B, Schlachetzki JC, Winkler J (2016) Oligodendroglia and myelin in neurodegenerative diseases: more than just bystanders? Mol Neurobiol 53:3046–3062. https://doi.org/10.1007/s12035-015-9205-3
Fernandez-Castaneda A, Gaultier A (2016) Adult oligodendrocyte progenitor cells—multifaceted regulators of the CNS in health and disease. Brain Behav Immun 57:1–7
Fu J, Tay SS, Ling EA, Dheen ST (2006) High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia 49:1027–1038. https://doi.org/10.1007/s00125-006-0153-3
Funfschilling U et al (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. https://doi.org/10.1038/nature11007
Ichihara Y, Doi T, Ryu Y, Nagao M, Sawada Y, Ogata T (2017) Oligodendrocyte progenitor cells directly utilize lactate for promoting cell cycling and differentiation. J Cell Physiol 232:986–995. https://doi.org/10.1002/jcp.25690
Kumar P, Raman T, Swain MM, Mishra R, Pal A (2017) Hyperglycemia-induced oxidative-nitrosative stress induces inflammation and neurodegeneration via augmented tuberous sclerosis complex-2 (TSC-2) activation in neuronal cells. Mol Neurobiol 54:238–254. https://doi.org/10.1007/s12035-015-9667-3
Lee Y et al (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. https://doi.org/10.1038/nature11314
Lopez Juarez A, He D, Richard LuQ (2016) Oligodendrocyte progenitor programming and reprogramming: toward myelin regeneration. Brain Res 1638:209–220
Morrison BM, Lee Y, Rothstein JD (2013) Oligodendroglia: metabolic supporters of axons. Trends Cell Biol 23:644–651
Naruse M, Ishizaki Y, Ikenaka K, Tanaka A, Hitoshi S (2016) Origin of oligodendrocytes in mammalian forebrains: a revised perspective. J Physiol Sci. https://doi.org/10.1007/s12576-016-0479-7
Nonneman A, Robberecht W, Van Den Bosch L (2014) The role of oligodendroglial dysfunction in amyotrophic lateral sclerosis. Neurodegener Dis Manag 4:223–239. https://doi.org/10.2217/nmt.14.21
Pellegrini D, Onor M, Degano I, Bramanti E (2014) Development and validation of a novel derivatization method for the determination of lactate in urine and saliva by liquid chromatography with UV and fluorescence detection. Talanta 130:280–287
Pellerin L (2003) Lactate as a pivotal element in neuron–glia metabolic cooperation. Neurochem Int 43:331–338
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94:1–14. https://doi.org/10.1111/j.1471-4159.2005.03168.x
Podbielska M, Banik NL, Kurowska E, Hogan EL (2013) Myelin recovery in multiple sclerosis: the challenge of remyelination. Brain Sci 3:1282–1324. https://doi.org/10.3390/brainsci3031282
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2:219–236
Richa R, Yadawa AK, Chaturvedi CM (2017) Hyperglycemia and high nitric oxide level induced oxidative stress in the brain and molecular alteration in the neurons and glial cells of laboratory mouse, Mus musculus. Neurochem Int 104:64–79
Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D (2011) Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 31:538–548. https://doi.org/10.1523/jneurosci.3516-10.2011
Rosa PM, Martins LAM, Souza DO, Quincozes-Santos A (2017) Glioprotective effect of resveratrol: an emerging therapeutic role for oligodendroglial cells. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0510-x
Rosafio K, Castillo X, Hirt L, Pellerin L (2016) Cell-specific modulation of monocarboxylate transporter expression contributes to the metabolic reprograming taking place following cerebral ischemia. Neuroscience 317:108–120
Rosko L, Smith VN, Yamazaki R, Huang JK (2018) Oligodendrocyte bioenergetics in health and disease. Neuroscientist. https://doi.org/10.1177/1073858418793077
Royland JE, Konat GW, Wiggins RC (1993) Myelin gene activation: a glucose sensitive critical period in development. J Neurosci Res 36:399–404. https://doi.org/10.1002/jnr.490360406
Selvarajah D, Tesfaye S (2006) Central nervous system involvement in diabetes mellitus. Curr Diab Rep 6:431–438
Shukla V, Shakya AK, Perez-Pinzon MA, Dave KR (2017) Cerebral ischemic damage in diabetes: an inflammatory perspective. J Neuroinflamm 14:21. https://doi.org/10.1186/s12974-016-0774-5
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791.
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS ONE 8:e60282. https://doi.org/10.1371/journal.pone.0060282
Tramontina AC et al (2012) High-glucose and S100B stimulate glutamate uptake in C6 glioma cells. Neurochem Res 37:1399–1408. https://doi.org/10.1007/s11064-012-0722-4
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Verdile G et al (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediat Inflamm 2015:105828. https://doi.org/10.1155/2015/105828
Wilkins A, Majed H, Layfield R, Compston A, Chandran S (2003) Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci 23:4967–4974
Yoon SY, Oh YJ (2015) Glucose levels in culture medium determine cell death mode in MPP(+)-treated dopaminergic neuronal cells. Exp Neurobiol 24:197–205. https://doi.org/10.5607/en.2015.24.3.197
Zhang Z, Yan J, Shi H (2013) Hyperglycemia as a risk factor of ischemic stroke. J Drug Metab Toxicol. https://doi.org/10.4172/2157-7609.1000153
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP)-Instituto Brasileiro de Neurociências (IBN Net) 01.06.0842-00, Universidade Federal do Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteçãao (INCTEN/CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Rosa, P.M., Meira, L.A.M., Souza, D.O. et al. High-glucose medium induces cellular differentiation and changes in metabolic functionality of oligodendroglia. Mol Biol Rep 46, 4817–4826 (2019). https://doi.org/10.1007/s11033-019-04930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04930-4