Abstract
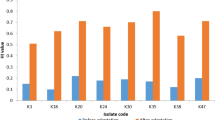
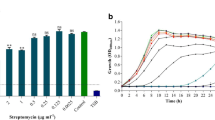
The increasing percentage of Pseudomonas aeruginosa strains that are resistant to multiple antibiotics is a global problem. The exposure of P. aeruginosa isolates to repeated sub lethal concentrations of biocides in hospitals and communities may be one of the causes leading to increased antibiotic resistance. Benzalkonium chloride (BAC) is widely used as disinfectant and preservative. This study investigated the effect of exposure of P. aeruginosa clinical isolates to sub lethal concentrations of BAC on their antibiotic resistance, growth process and biofilm formation. The collected 43 P. aeruginosa clinical isolates were daily subjected to increasing sub lethal concentrations of BAC. The effect of adaptation on antibiotic resistance, growth process, cell surface hydrophobicity and biofilm formation of P. aeruginosa isolates were examined. Interestingly, Most P. aeruginosa isolates adapted to BAC showed an increase in antibiotic resistance and 66% of the isolates showed retardation of growth, 63% showed increased cell surface hydrophobicity and 23.5% exhibited enhanced biofilm formation by crystal violet assay. To define whether the effect of BAC adaptation on biofilm production was manifested at the transcriptional level, quantitative RT-PCR was used. We found that 60% of the tested isolates showed overexpression of ndvB biofilm gene. More efforts are required to diminish the increasing use of BAC to avoid bacterial adaptation to this biocide with subsequent retardation of growth and enhanced biofilm formation which could lead to antibiotic resistance and treatment failure of infections caused by this opportunistic pathogen.

Similar content being viewed by others
References
Abdelaziz A, El Banna T, Sonbol F, Gamaledin N, Maghraby G (2014) Optimization of niosomes for enhanced antibacterial activity and reduced bacterial resistance: in vitro and in vivo evaluation. Exp Opin Drug Deliv 12:163–180
El Banna T, Sonbol F, Abd El Aziz A, Elekhnawy E (2015) Characterization of vancomycin resistant Staphylococcus aureus in Tanta university hospital. Int J Curr Microbiol App Sci 4:1–11
El Banna T, Sonbol F, Abd El Aziz A, Elekhnawy E (2018) Prevalence and antimicrobial susceptibility of vancomycin resistant staphylococci in an Egyptian university hospital. J Med Microb Diagn 7:1–8
Deep A, Chaudhary A, Gupta V (2011) Quorum sensing and bacterial pathogenicity: from molecules to disease. J Lab Phys 3:4–11
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS 136:1–51
Ebrahimi A, Hemati M, Shabanpour Z, Dehkordi S, Bahadoran S, Lotfalian S et al (2015) Effects of benzalkonium chloride on planktonic growth and biofilm formation by animal bacterial pathogens. Jundishapur J Microbiol 8:e16058
Houari A, Di Martino P (2007) Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Lett App Microbiol 45:652–656
Gerba CP (2015) Quaternary ammonium biocides: efficacy in application. App Environ Microbiol 81:464–469
MacFaddin JF (1976) Biochemical tests for identification of medical bacteria. Williams & Wilkins Co., Baltimore
Clinical and Laboratory Standards Institute (CLSI) (2017) Performance standards for antimicrobial susceptibility testing. Wayne, Pennsylvania
Moen B, Rudi K, Bore E, Langsrud S (2012) Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of Escherichia coli with inheritable characteristics. Int J Mol Sci 13:4101–4123
Sonbol F, El-Banna T, AbdEl-Aziz A, El-Ekhnawy E (2019) Impact of triclosan aadaptation on membrane properties, efflux and antimicrobial resistance of Escherichia coli clinical isolates. J Appl Microbiol 126:730–739
Soumet C, Meheust D, Pissavin M, Le Grandois P, Fremaux B, Feurer M et al (2016) Reduced susceptibilities to biocides and resistance to antibiotics in food-associated bacteria following exposure to quaternary ammonium compounds. J Appl Microbiol 121:1275–1281
Williams DN, Ehrman SH, Holoman TRP (2006) Evaluation of the microbial growth response to inorganic nanoparticles. J Nanoobiotechnol 4:1–8
Rosenberg M (1984) Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol Lett 22:289–295
Sonbol F, El-Banna T, Abd El-Aziz A, El-Ekhnawy E (2018) Biological characters of vancomycin resistant Staphylococcus aureus isolates from a university hospital in Egypt. Arch Clin Microbial 9:72
Yang B, Lei Z, ZhaoY Ahmed S, Wang C, Zhang S et al (2017) Combination susceptibility testing of common antimicrobials in vitro and the effects of sub-MIC of antimicrobials on Staphylococcus aureus biofilm formation. Front Microbiol 8:2125
Trafny EA, Lewandowski R, Zawistowska-Marciniak I, Stepińska M (2013) Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metal working fluids. World J Microbiol Biotechnol 29:1635–1643
Lu S, Tian Q, Zhao W, Hu B (2017) Evaluation of the potential of five housekeeping genes for identification of quarantine Pseudomonas syringae. J Phytopathol 165:73–81
Beaudoin T, Zhang L, Hinz AJ, Parr CJ, Mah TF (2013) The biofilmspecific antibiotic resistance gene ndvB is important for expression of ethanol oxidation genes in Pseudomonas aeruginosa biofilms. J Bacteriol 194:3128–3136
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Fernández-Cuenca F, Tomás M, Caballero-Moyano F, Bou G, Martínez L, Vila J et al (2015) Reduced susceptibility to biocides in Acinetobacter baumannii: association with resistance to antimicrobials, epidemiological behaviour, biological cost and effect on the expression of genes encoding porins and efflux pumps. J Antimicrob Chemother 70:3222–3229
Cabot G, Zamorano L, Moyà B, Juan C, Navas A, Blázquez J, Oliver A (2016) evolution of Pseudomonas aeruginosa antimicrobial resistance and fitness under low and high mutation rates. J Atimicrob Agents Chem 60:1767–1778
Abdelaziz A, Sonbol S, Elbanna T, El-Ekhnaw E (2018) Exposure to sublethal concentrations of benzalkonium chloride induces antimicrobial resistance and cellular changes in Klebsiellae pneumoniae clinical isolates. Microb Drug Resist. https://doi.org/10.1089/mdr.2018.0235
Capita R, Riesco-Peláez F, Alonso-Hernando A, Alonso-Calleja C (2014) Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl Environ Microbiol 80(4):1268–1280
Gallardo-Moreno AM, González-Martín ML, Pérez-Giraldo C, Garduno E, Bruque JM, Gómez-García AC (2002) Thermodynamic analysis of growth temperature dependence in the adhesion of Candida parapsilosis to polystyrene. Appl Environ Microbiol 68:2610–2613
Heilmann C (2011) Adhesion mechanisms of staphylococci. Adv Exp Med Biol 715:105–123
Rodrigues DF, Elimelech M (2009) Role of type 1 fimbriae and mannose in the development of Escherichia coli K12 biofilm: from initial cell adhesion to biofilm formation. Biofouling 25:401–411
Andersson S, Kuttuva G, Carl RL, Dalhammar G (2008) Biofilm formation and interactions of bacterial strains found in wastewater treatment systems. FEMS Microbiol Lett 283:83–90
Krasowska A, Sigler K (2014) How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol 4:112
Elbanna T, Sonbol S, Abdelaziz A, Elekhnawy E (2015) Increased biofilm production associated with increasing vancomycin MICs among vancomycin resistant staphylococci. Inventi 4:1–4
Hamadi F, Latrache H, Zahir H, Elghmari A, Timinouni M, Ellouali M (2008) The relation between Escherichia coli surface functional groups composition and their physicochemical properties. Braz J Microbiol 39:10–15
Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122
Lewis K (2007) Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56
Allison KR, Brynildsen MP, Collins JJ (2011) Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220
Greulich P, Scott M, Evans MR, Allen RJ (2015) Growth-dependent bacterial susceptibility to ribosome-targeting antibiotics. Mol Syst Biol 11:796
Millar MR, Pike J (1992) Bactericidal activity of antimicrobial agents against slowly growing Helicobacter pylori. Antimicrob Agents Chem 36:185–187
Beceiro A, Tomás M, Bou G (2013) Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev 26:185–230
Machado I, Graça J, Sousa AM, Lopes SP, Pereira MO (2011) Effect of antimicrobial residues on early adhesion and biofilm formation by wild-type and benzalkonium chloride-adapted Pseudomonas aeruginosa. Biofouling 27:1151–1159
Pagedar A, Singh J, Batish VK (2012) Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. J Dairy Res 79:383–389
Moretro T, Hermansen L, Holck AL, Sidhu MS, Rudi K, Langsrud S (2003) Biofilm formation and the presence of the intercellular adhesion locus ICA among staphylococci from food and food processing environments. Appl Environ Microbiol 69:5648–5655
Milisavljevic V, Tran LP, Batmalle C, Bootsma H (2008) Benzyl alcohol and ethanol can enhance the pathogenic potential of clinical Staphylococcus epidermidis strains. Am J Infect Control 36:552–558
Wang H, Cheng H, Wang F, Wei D, Wang X (2010) An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J Microbiol Methods 82:330–333
Grela E, Ząbek A, Grabowiecka A (2015) Interferences in the optimization of the MTT assay for viability estimation of Proteus mirabilis. Avicenna J Med Biotech 7:159–167
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Banna, T., Abd El-Aziz, A., Sonbol, F. et al. Adaptation of Pseudomonas aeruginosa clinical isolates to benzalkonium chloride retards its growth and enhances biofilm production. Mol Biol Rep 46, 3437–3443 (2019). https://doi.org/10.1007/s11033-019-04806-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04806-7