Abstract
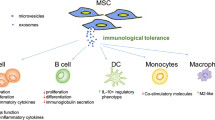
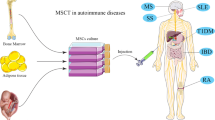
In autoimmune disease body’s own immune system knows healthy cells as undesired and foreign cells. Over 80 types of autoimmune diseases have been recognized. Currently, at clinical practice, treatment strategies for autoimmune disorders are based on relieving symptoms and preventing difficulties. In other words, there is no effective and useful therapy up to now. It has been well-known that mesenchymal stem cells (MSCs) possess immunomodulatory effects. This strongly suggests that MSCs might be as a novel modality for treatment of autoimmune diseases. Supporting this notion a few preclinical and clinical studies indicate that MSCs ameliorate autoimmune disorders. Interestingly, it has been found that the beneficial effects of MSCs in autoimmune disorders are not relying only on direct cell-to-cell communication but on their capability to produce a broad range of paracrine factors including growth factors, cytokines and extracellular vehicles (EVs). EVs are multi-signal messengers that play a serious role in intercellular signaling through carrying cargo such as mRNA, miRNA, and proteins. Numerous studies have shown that MSC-derived EVs are able to mimic the effects of the cell of origin on immune cells. In this review, we discuss the current studies dealing with MSC-based therapies in autoimmune diseases and provide a vision and highlight in order to introduce MSC-derived EVs as an alternative and emerging modality for autoimmune disorders.



Similar content being viewed by others
References
Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J Intern Med 278(4):369–395
Ray S, Sonthalia N, Kundu S, Ganguly S (2012) Autoimmune disorders: an overview of molecular and cellular basis in today’s perspective. J Clin Cell Immunol S 10:003
Abbas AK, Lichtman AH, Pillai S (2014) Cellular and molecular immunology E-book: Elsevier Health Sciences
Jancar S, Crespo MS (2005) Immune complex-mediated tissue injury: a multistep paradigm. Trends Immunol 26(1):48–55
Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M (2010) The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 6(5):280
Roeleveld DM, Koenders MI (2015) The role of the Th17 cytokines IL-17 and IL-22 in rheumatoid arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 74(1):101–107
Zhang H-L, Zheng X-Y, Zhu J (2013) Th1/Th2/Th17/Treg cytokines in Guillain–Barré syndrome and experimental autoimmune neuritis. Cytokine Growth Factor Rev 24(5):443–453
Li Y-F, Zhang S-X, Ma X-W, Xue Y-L, Gao C, Li X-Y (2017) Levels of peripheral Th17 cells and serum Th17-related cytokines in patients with multiple sclerosis: a meta-analysis. Multiple Scler Relat Disord 18:20–25
Nicoletti F, Créange A, Orlikowski D, Bolgert F, Mangano K, Metz C et al (2005) Macrophage migration inhibitory factor (MIF) seems crucially involved in Guillain–Barré syndrome and experimental allergic neuritis. J Neuroimmunol 168(1–2):168–174
Benedek G, Meza-Romero R, Jordan K, Zhang Y, Nguyen H, Kent G et al (2017) MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc Natl Acad Sci 114(40):E8421–E9
Fagone P, Mazzon E, Cavalli E, Bramanti A, Petralia MC, Mangano K et al (2018) Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: in silico and in vivo evidences. J Neuroimmunol 322:46–56
Karo-Atar D, Bitton A, Benhar I, Munitz A (2018) Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: in allergy and beyond. BioDrugs 32:1–20
Fagone P, Mangano K, Pesce A, Portale TR, Puleo S, Nicoletti F (2016) Emerging therapeutic targets for the treatment of hepatic fibrosis. Drug Discov Today 21(2):369–375
Barcellini W, Rizzardi G, Borghi M, Nicoletti F, Fain C, Del Papa N et al (1996) In vitro type-1 and type-2 cytokine production in systemic lupus erythematosus: lack of relationship with clinical disease activity. Lupus 5(2):139–145
Nicoletti F, Di Marco R, Patti F, Reggio E, Nicoletti A, Zaccone P et al (1998) Blood levels of transforming growth factor-beta 1 (TGF-β1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-β1b). Clin Exp Immunol 113(1):96–99
Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N et al (2009) The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: the elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol 207(1–2):101–106
Nadkarni S, Mauri C, Ehrenstein MR (2007) Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J Exp Med 204(1):33–39
Wu Q, Wang Q, Mao G, Dowling CA, Lundy SK, Mao-Draayer Y (2017) Dimethyl fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol. https://doi.org/10.4049/jimmunol.1601532
Lai Y, Dong C (2015) Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int Immunol 28(4):181–188
Fragoulis GE, Siebert S, McInnes IB (2016) Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Ann Rev Med 67:337–353
Scharl M, Vavricka R, Rogler G (2013) New anti-cytokines for IBD: what is in the pipeline? Curr Drug Targets 14(12):1405–1420
Ramos-Casals M, Diaz-Lagares C, Cuadrado M-J, Khamashta MA, Group BS (2010) Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev 9(3):188–193
Zintzaras E, Voulgarelis M, Moutsopoulos HM (2005) The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med 165(20):2337–2344
Pérez-De-Lis M, Retamozo S, Flores-Chávez A, Kostov B, Perez-Alvarez R, Brito-Zerón P et al (2017) Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry). Expert Opin Drug Saf 16(11):1255–1271
Kalden JR, Schulze-Koops H (2017) Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol 13(12):707
Tyndall A (2011) Successes and failures of stem cell transplantation in autoimmune diseases. ASH Educ Program Book 2011(1):280–284
Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M et al (2015) Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant 24(12):2615–2627
Castro-Manrreza ME, Montesinos JJ (2015) Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. https://doi.org/10.1155/2015/394917
Blanco B, Herrero-Sánchez MdC, Rodríguez-Serrano C, García-Martínez ML, Blanco JF, Muntión S et al (2016) Immunomodulatory effects of bone marrow versus adipose tissue-derived mesenchymal stromal cells on NK cells: implications in the transplantation setting. Eur J Haematol 97(6):528–537
Amiri F, Jahanian-Najafabadi A, Roudkenar MH (2015) In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments. Cell Stress Chaperones 20(2):237–251
Roushandeh AM, Bahadori M, Roudkenar MH (2017) Mesenchymal stem cell-based therapy as a new horizon for kidney injuries. Arch Med Res 48(2):133–146
Fontaine MJ, Shih H, Schäfer R, Pittenger MF (2016) Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus Med Rev 30(1):37–43
Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G (2012) Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant 27(8):3037–3042
Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 3:359
Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK (2009) Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 38(1):215–224
Abbasi-Malati Z, Roushandeh AM, Kuwahara Y, Roudkenar MH (2018) Mesenchymal stem cells on horizon: a new arsenal of therapeutic agents. Stem Cell Rev Rep 14:484–499
Bassi ÊJ, de Almeida DC, Moraes-Vieira PMM, Câmara NOS (2012) Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev Rep 8(2):329–342
Vianello F, Dazzi F (2008) Mesenchymal stem cells for graft-versus-host disease: a double edged sword? Leukemia 22(3):463-465
Halabian R, Tehrani HA, Jahanian-Najafabadi A, Roudkenar MH (2013) Lipocalin-2-mediated upregulation of various antioxidants and growth factors protects bone marrow-derived mesenchymal stem cells against unfavorable microenvironments. Cell Stress Chaperones 18(6):785–800
Kiani AA, Kazemi A, Halabian R, Mohammadipour M, Jahanian-Najafabadi A, Roudkenar MH (2013) HIF-1α confers resistance to induced stress in bone marrow-derived mesenchymal stem cells. Arch Med Res 44(3):185–193
Wong RS (2011) Mesenchymal stem cells: angels or demons? BioMed Res Int. https://doi.org/10.1155/2011/459510
Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2(4):313–319
Short B, Brouard N, Driessen R, Simmons P (2001) Prospective isolation of stromal progenitor cells from mouse BM. Cytotherapy 3(5):407–408
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36(4):568–584
Amiri F, Halabian R, Salimian M, Shokrgozar MA, Soleimani M, Jahanian-Najafabadi A et al (2014) Induction of multipotency in umbilical cord-derived mesenchymal stem cells cultivated under suspension conditions. Cell Stress Chaperones 19(5):657–666
Amiri F, Halabian R, Dehgan Harati M, Bahadori M, Mehdipour A, Mohammadi Roushandeh A et al (2015) Positive selection of Wharton’s jelly-derived CD105 + cells by MACS technique and their subsequent cultivation under suspension culture condition: a simple, versatile culturing method to enhance the multipotentiality of mesenchymal stem cells. Hematology 20(4):208–216
Troyer DL, Weiss ML (2008) Concise review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3):591–599
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25(11):2739–2749
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I et al (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67(10):1187–1194
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH (2008) Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57(7):1759–1767
Le Blanc K, Ringden O (2007) Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 262(5):509–525
Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D (2010) Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther 1(1):2
Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW et al (2005) T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 12(1):47–57
Bradley JA, Bolton EM, Pedersen RA (2002) Stem cell medicine encounters the immune system. Nat Rev Immunol 2(11):859
Götherström C (2007) Immunomodulation by multipotent mesenchymal stromal cells. Transplantation 84(1):S35–S37
De Miguel P, Fuentes-Julian M, Blazquez-Martinez S, Pascual C AY, Aller A, Arias M et al (2012) Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12(5):574–591
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822
Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K et al (2014) Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6(5):552
Castro-Manrreza ME (2016) Participation of mesenchymal stem cells in the regulation of immune response and cancer development. Boletín Médico Del Hospital Infantil de México (English Edition) 73(6):380–387
Le Blanc K, Tammik L, Sundberg B, Haynesworth S, Ringden O (2003) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57(1):11–20
Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A et al (2006) Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24(2):386–398
Glennie S, Soeiro I, Dyson PJ, Lam EW-F, Dazzi F (2005) Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105(7):2821–2827
Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT et al (2011) Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res 7(1):66–74
Lee H-J, Kim S-N, Jeon M-S, Yi T, Song SU (2017) ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci Rep 7:44486
Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon S-o et al (2016) Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo-and autoimmunity in the eye. Proc Natl Acad Sci 113(1):158–163
Yan L, Zheng D, Xu R (2018) Critical role of TNF signaling in mesenchymal stem cell-based therapy for autoimmune and inflammatory diseases. Front Immunol 9:1658
Angulski AB, Capriglione LG, Batista M, Marcon BH, Senegaglia AC, Stimamiglio MA et al (2017) The protein content of extracellular vesicles derived from expanded human umbilical cord blood-derived CD133+ and human bone marrow-derived mesenchymal stem cells partially explains why both sources are advantageous for regenerative medicine. Stem Cell Rev Rep 13(2):244–257
Sagini K, Costanzi E, Emiliani C, Buratta S, Urbanelli L (2018) Extracellular vesicles as conveyors of membrane-derived bioactive lipids in immune system. Int J Mol Sci 19(4):1227
Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Álvarez V, Tarazona R et al (2014) Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol 5:556
de Araújo Farias V, Carrillo-Gálvez AB, Martín F, Anderson P (2018) TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev 43:25–37
Gebler A, Zabel O, Seliger B (2012) The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med 18(2):128–134
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8(9):726
Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S et al (2009) Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett 126(1–2):37–42
Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE (2006) Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 177(4):2080–2087
Liu W-h, Liu J-j, Wu J, Zhang L-l, Liu F, Yin L et al (2013) Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS ONE 8(1):e55487
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24(1):74–85
Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F et al (2008) Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26(1):151–162
Zhao Q, Ren H, Han Z (2016) Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother 2(1):3–20
He M, Shi X, Yang M, Yang T, Li T, Chen J (2019) Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR signaling to inhibit the proliferation of reactive astrocytes induced by hypoxic-ischemic brain damage. Exp Neurol 311:15–32
Mammana S, Bramanti P, Mazzon E, Cavalli E, Basile MS, Fagone P et al (2018) Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget 9(9):8263
Donia M, Mangano K, Amoroso A, Mazzarino MC, Imbesi R, Castrogiovanni P et al (2009) Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+ CD25+ Foxp3+ regulatory T cells. J Autoimmun 33(2):135–140
Faustman D, Davis M (2010) TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov 9(6):482
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E et al (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106(5):1755–1761
Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J et al (2005) Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen-induced arthritis. Arthritis Rheum 52(5):1595–1603
González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M (2009) Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 60(4):1006–1019
Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH et al (2008) Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol 153(2):269–276
Villatoro AJ, Fernández V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA (2015) Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. BioMed Res Int. https://doi.org/10.1155/2015/527926
Bassi ÊJ, Moraes-Vieira PM, Sá CSM, Almeida DC, Vieira LM, Cunha CS et al (2012) Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. https://doi.org/10.2337/db11-0844
Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E et al (2009) Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol 182(10):5994–6002
Jang E, Jeong M, Kim S, Jang K, Kang B-K, Lee DY et al (2016) Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T-cell development. Cell Transplant 25(1):1–15
Youd M, Blickarz C, Woodworth L, Touzjian T, Edling A, Tedstone J et al (2010) Allogeneic mesenchymal stem cells do not protect NZB × NZW F1 mice from developing lupus disease. Clin Exp Immunol 161(1):176–186
Linero I, Chaparro O (2014) Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 9(9):e107001
Yu B, Zhang X, Li X (2014) Exosomes derived from mesenchymal stem cells. Int J Mol Sci 15(3):4142–4157
Baraniak PR, McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regenerat Med 5(1):121–143
Shimada Y, Minna JD (2017) Exosome mediated phenotypic changes in lung cancer pathophysiology. Transl Cancer Res 6(S6):S1040–S1042
Simons M, Raposo G (2009) Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol 21(4):575–581
Rad F, Pourfathollah AA, Yari F, Mohammadi S, Kheirandish M (2016) Microvesicles preparation from mesenchymal stem cells. Med J IR Iran 30:398
Nassar W, El-Ansary M, Aziz MA, El-Hakim E (2015) Extracellular vesicles: fundamentals and clinical relevance. Egypt J Intern Med 27(1):1
Pan B-T, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33(3):967–978
Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23(5):812–823
Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M (2015) Isolation of extracellular vesicles: determining the correct approach. Int J Mol Med 36(1):11–17
Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjøt L et al (2014) Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles 3(1):25011
Van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64(3):676–705
Simpson RJ, Kalra H, Mathivanan S (2012) ExoCarta as a resource for exosomal research. J Extracell Vesicles 1(1):18374
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383
Beer KB, Wehman AM (2017) Mechanisms and functions of extracellular vesicle release in vivo—what we can learn from flies and worms. Cell Adhes Migr 11(2):135–150
Tricarico C, Clancy J, D’Souza-Schorey C (2017) Biology and biogenesis of shed microvesicles. Small GTPases 8(4):220–232
Katsuda T, Kosaka N, Takeshita F, Ochiya T (2013) The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 13(10–11):1637–1653
Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L et al (2010) Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE 5(7):e11803
Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G (2012) Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 22(5):758–771
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F et al (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20(5):1053–1067
Skalnikova HK (2013) Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 95(12):2196–2211
Kim H-S, Choi D-Y, Yun SJ, Choi S-M, Kang JW, Jung JW et al (2011) Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 11(2):839–849
Zhang H-C, Liu X-B, Huang S, Bi X-Y, Wang H-X, Xie L-X et al (2012) Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev 21(18):3289–3297
Park C, Huang JZ, Ji JX, Ding Y (2013) Segmentation, inference and classification of partially overlapping nanoparticles. IEEE Trans Pattern Anal Mach Intell 35(3):1–1
Yáñez-Mó M, Siljander PR-M, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI et al (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4(1):27066
Kalra H, Drummen GP, Mathivanan S (2016) Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 17(2):170
Lin J, Li J, Huang B, Liu J, Chen X, Chen X-M et al (2015) Exosomes: novel biomarkers for clinical diagnosis. Sci World J. https://doi.org/10.1155/2015/657086
Ferguson SW, Nguyen J (2016) Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Controlled Release 228:179–190
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654
Ratajczak MZ, Ratajczak J (2016) Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin Transl Med 5(1):7
Quesenberry PJ, Aliotta J, Deregibus MC, Camussi G (2015) Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res Ther 6(1):153
Quesenberry PJ, Goldberg LR, Aliotta JM, Dooner MS, Pereira MG, Wen S et al (2014) Cellular phenotype and extracellular vesicles: basic and clinical considerations. Stem Cells Dev 23(13):1429–1436
Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekström K et al (2016) Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. https://doi.org/10.1155/2016/1073140
Nawaz M, Fatima F (2017) Extracellular vesicles, tunneling nanotubes, and cellular interplay: synergies and missing links. Front Mol Biosci 4:50
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J et al (2015) LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med 13(1):308
Ti D, Hao H, Fu X, Han W (2016) Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci 59(12):1305–1312
Di Rocco G, Baldari S, Toietta G (2016) Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem Cells Int. https://doi.org/10.1155/2016/5029619
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I et al (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4(1):26316
Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C et al (2014) Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 33(5):1055–1063
Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M (2013) The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transpl 22(2):369–379
Henao Agudelo JS, Braga TT, Amano MT, Cenedeze MA, Cavinato RA, Peixoto-Santos AR et al (2017) Mesenchymal stromal cell-derived microvesicles regulate an internal pro-inflammatory program in activated macrophages. Front Immunol 8:881
Shigemoto-Kuroda T, Oh JY, Kim D-k, Jeong HJ, Park SY, Lee HJ et al (2017) MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep 8(5):1214–1225
Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C et al (2018) Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8(5):1399
Kordelas L, Rebmann V, Ludwig A, Radtke S, Ruesing J, Doeppner T et al (2014) MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28(4):970
Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P et al (2016) Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 59(2):325–333
Sharma J, Hampton JM, Valiente GR, Wada T, Steigelman H, Young MC et al (2017) Therapeutic development of mesenchymal stem cells or their extracellular vesicles to inhibit autoimmune-mediated inflammatory processes in systemic lupus erythematosus. Front Immunol 8:526
Hai B, Shigemoto-Kuroda T, Zhao Q, Lee RH, Liu F (2018) Inhibitory effects of iPSC-MSCs and their extracellular vesicles on the onset of sialadenitis in a mouse model of Sjögren’s Syndrome. Stem Cells Int. https://doi.org/10.1155/2018/2092315
Soundara Rajan T, Giacoppo S, Diomede F, Bramanti P, Trubiani O, Mazzon E (2017) Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int J Immunopathol Pharmacol 30(3):238–252
Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM (2018) Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem 119(11):9433–9443
Rajan TS, Giacoppo S, Diomede F, Ballerini P, Paolantonio M, Marchisio M et al (2016) The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci Rep 6:38743
Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ, Otero-Ortega L, Feliú A, Gómez-de Frutos M et al (2018) Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 13(9):e0202590
Chen Z, Wang H, Xia Y, Yan F, Lu Y (2018) Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol 201(8):2472–2482
Zhu L, Huang X, Yu W, Chen H, Chen Y, Dai Y (2018) Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50(2):e12871
Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid A-A, Mardani K (2012) Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett 147(1–2):47–54
Jacquelin S, Licata F, Dorgham K, Hermand P, Poupel L, Guyon E et al (2013) CX3CR1 reduces Ly6Chigh-monocyte motility within, and release from the bone marrow after chemotherapy in mice. Blood. https://doi.org/10.1182/blood-2013-01-480749
Hidalgo-Garcia L, Galvez J, Rodriguez-Cabezas ME, Anderson PO (2018) Can a conversation between mesenchymal stromal cells and macrophages solve the crisis in the inflamed intestine? Front Pharmacol 9:179
Jaimes Y, Naaldijk Y, Wenk K, Leovsky C, Emmrich F (2017) Mesenchymal stem cell-derived microvesicles modulate lipopolysaccharides-induced inflammatory responses to microglia cells. Stem Cells 35(3):812–823
Bruno S, Deregibus MC, Camussi G (2015) The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett 168(2):154–158
Favaro E, Deregibus M, Camussi E, Granata R, Ghigo E, Cavallo PP et al (2012) Mesenchymal stem cells-derived microvesicles modulate cellular immune response to islet antigen GAD in type 1 diabetes. 15th International & 14th European Congress of Endocrinology; BioScientifica
Tamura R, Uemoto S, Tabata Y (2016) Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm Regenerat 36(1):26
Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK (2013) Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 23(11):1233–1244
Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noël D (2017) Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci 18(4):889
Perez-Hernandez J, Redon J, Cortes R (2017) Extracellular vesicles as therapeutic agents in systemic lupus erythematosus. Int J Mol Sci 18(4):717
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L et al (2017) Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep 7(1):4323
Acknowledgements
Part of this study was supported by Guilan University of Medical Sciences (Grant No: IR.GUMS.REC.1396.343).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rad, F., Ghorbani, M., Mohammadi Roushandeh, A. et al. Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Mol Biol Rep 46, 1533–1549 (2019). https://doi.org/10.1007/s11033-019-04588-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04588-y