Abstract
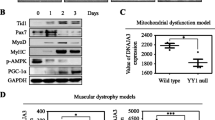
In order to investigate the mechanism of suppressor of cytokine signaling 2 (SOCS2) on mitochondrial biogenesis, RNA interference and over-expression plasmid vectors of SOCS2 were used to transfect murine skeletal muscle C2C12 cells. Results demonstrated that over-expression of SOCS2 inhibited the differentiation of C2C12 cells, and reduced the expression of MyHC, MyoD and MyoG while elevated the protein expression of MSTN. Meanwhile the expression of PGC-1α, MDH, CPT-1 were significantly elevated in the RNA interference of SOCS2 group which were decreased in SOCS2 overexpression group. However, there was no change on the expression of UCP1 in both two groups. JC-1 dyeing showed overexpression of SOCS2 decreased the mitochondrial membrane potential and results of immunofluorescence, real-time PCR and western blotting indicated the increase expression of Cyt c, while interference SOCS2 had the opposite effects in C2C12 cells. Moreover, interference of SOCS2 elevated the p38 phosphorylation level then further increased the phosphorylation of ATF2, whereas overexpression of SOCS2 alleviated this phenomenon. Taken together, our observations indicated that SOCS2 could suppress myotube formation, act as an anti-regulator of mitochondria biogenesis via inhibiting p38 MAPK signal pathway.








Similar content being viewed by others
References
Greenhalgh CJ, Rico-Bautista E, Lorentzon M, Thaus AL, Morgan PO, Willson TA, Zervoudakis P, Metcalf D, Street I, Nicola NA (2005) SOCS2 negatively regulates growth hormone action in vitro and in vivo. J Clin Investig 115:397–406
Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, Fabri LJ, Zhang J-G, Martin HM, Willson TA (2002) Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem 277:40181–40184
Rico-Bautista E, Flores-Morales A, Fernández-Pérez L (2006) Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev 17:431–439
Kato H, Nomura K, Osabe D, Shinohara S, Mizumori O, Katashima R, Iwasaki S, Nishimura K, Yoshino M, Kobori M (2006) Association of single-nucleotide polymorphisms in the suppressor of cytokine signaling 2 (SOCS2) gene with type 2 diabetes in the Japanese. Genomics 87:446–458
Puff R, Dames P, Weise M, Göke B, Parhofer K, Lechner A (2010) No non-redundant function of suppressor of cytokine signaling 2 in insulin producing β-cells. Islets 2:252–257
Zadjali F, Santana-Farre R, Vesterlund M, Carow B, Mirecki-Garrido M, Hernandez–Hernandez I, Flodström-Tullberg M, Parini P, Rottenberg M, Norstedt G (2012) SOCS2 deletion protects against hepatic steatosis but worsens insulin resistance in high-fat-diet-fed mice. FASEB J 26:3282–3291
Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trend Endocrinol Metab 23:459–466
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc Res 79:208–217
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122
Shi T, Wang F, Stieren E, Tong Q (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280:13560–13567
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283:1482–1488
Zarubin T, Jiahuai H (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res 15:11–18
Ruiz-Bonilla V, Perdiguero E, Gresh L, Serrano AL, Zamora M, Sousa-Victor P, Jardí M, Wagner EF, Muñoz-Cánoves P (2008) Efficient adult skeletal muscle regeneration in mice deficient in p38β, p38γ and p38δ MAP kinases. Cell Cycle 7:2208–2214
Ho RC, Alcazar O, Fujii N, Hirshman MF, Goodyear LJ (2004) p38γ MAPK regulation of glucose transporter expression and glucose uptake in L6 myotubes and mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol 286:R342–R349
Zetser A, Gredinger E, Bengal E (1999) p38 Mitogen-activated protein kinase pathway promotes skeletal muscle differentiation participation of the MEF2C transcription factor. J Biol Chem 274:5193–5200
Röckl KS, Witczak CA, Goodyear LJ (2008) Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 60:145–153
Knutti D, Kressler D, Kralli A (2001) Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci USA 98:9713–9718
Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang C-Y, Krauss S, Mootha VK, Lowell BB, Spiegelman BM (2001) Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol Cell 8:971–982
Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z (2005) Exercise stimulates PGC-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280:19587–19593
Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S (2004) p38 Mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24:3057–3067
Wright DC, Geiger PC, Han D-H, Jones TE, Holloszy JO (2007) Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282:18793–18799
Hu J, Winqvist O, Flores-Morales A, Wikström A-C, Norstedt G (2009) SOCS2 influences LPS induced human monocyte-derived dendritic cell maturation. PLoS One 4:e7178
Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK (2005) Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54:2939–2945
Lim J, Lee J, Suh Y, Kim W, Song J, Jung M (2006) Mitochondrial dysfunction induces aberrant insulin signalling and glucose utilisation in murine C2C12 myotube cells. Diabetologia 49:1924–1936
Inaba M, Saito H, Fujimoto M, Sumitani S, Ohkawara T, Tanaka T, Kouhara H, Kasayama S, Kawase I, Kishimoto T (2005) Suppressor of cytokine signaling 1 suppresses muscle differentiation through modulation of IGF-I receptor signal transduction. Biochem Biophys Res Commun 328:953–961
Spangenburg EE, Brown DA, Johnson MS, Moore RL (2006) Exercise increases SOCS-3 expression in rat skeletal muscle: potential relationship to IL-6 expression. J Physiol 572:839–848
Sadowski CL, Wheeler TT, Wang L-H, Sadowski HB (2001) GH regulation of IGF-I and suppressor of cytokine signaling gene expression in C2C12 skeletal muscle cells. Endocrinology 142:3890–3900
Miller ME, Michaylira CZ, Simmons JG, Ney DM, Dahly EM, Heath JK, Lund PK (2004) Suppressor of cytokine signaling-2: a growth hormone-inducible inhibitor of intestinal epithelial cell proliferation. Gastroenterology 127:570–581
Fruchtman S, Simmons JG, Michaylira CZ, Miller ME, Greenhalgh CJ, Ney DM, Lund PK (2005) Suppressor of cytokine signaling-2 modulates the fibrogenic actions of GH and IGF-I in intestinal mesenchymal cells. Am J Physiol Gastrointest Liver Physiol 289:G342–G350
Czubryt MP, McAnally J, Fishman GI, Olson EN (2003) Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100:1711–1716
Kraft CS, LeMoine CM, Lyons CN, Michaud D, Mueller CR, Moyes CD (2006) Control of mitochondrial biogenesis during myogenesis. Am J Physiol Cell Physiol 290:C1119–C1127
Philp A, Belew MY, Evans A, Pham D, Sivia I, Chen A, Schenk S, Baar K (2011) The PGC-1α-related coactivator promotes mitochondrial and myogenic adaptations in C2C12 myotubes. Am J Physiol Regul Integr Comp Physiol 301:R864–R872
Vega RB, Huss JM, Kelly DP (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876
Liang H, Ward WF (2006) PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30:145–151
Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM (2002) Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277:1645–1648
Hammarstedt A, Jansson P-A, Wesslau C, Yang X, Smith U (2003) Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys Res Commun 301:578–582
Mitra R, Nogee DP, Zechner JF, Yea K, Gierasch CM, Kovacs A, Medeiros DM, Kelly DP, Duncan JG (2012) The transcriptional coactivators, PGC-1α and β, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol 52:701–710
Oba T, Yasukawa H, Hoshijima M, Sasaki K-i, Futamata N, Fukui D, Mawatari K, Nagata T, Kyogoku S, Ohshima H (2012) Cardiac-specific deletion of SOCS-3 prevents development of left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 59:838–852
Luo B, Zou T, Lu N, Chai F, Ye X, Wang Y, Qi Y (2011) Role of suppressor of cytokine signaling 3 in lipid metabolism: analysis based on a phage-display human liver cDNA library. Biochem Biophys Res Commun 416:39–44
Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B (2001) UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504:82–106
Kholmukhamedov A, Schwartz JM, Lemasters JJ (2013) Isolated mitochondria infusion mitigates ischemia-reperfusion injury of the liver in rats: mitotracker probes and mitochondrial membrane potential. Shock 39:543
Pelletier H, Kraut J (1992) Crystal structure of a complex between electron transfer partners, cytochrome c peroxidase and cytochrome c. Science 258:1748–1755
Gray HB, Winkler JR (1996) Electron transfer in proteins. Annu Rev Biochem 65:537–561
Herlaar E, Brown Z (1999) p38 MAPK signaling cascades in inflammatory disease. Mol Med Today 5:439–447
Acknowledgments
This work was supported by the National Nature Science Foundation of China (31172185).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lu Gan and Zhenjiang Liu have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Gan, L., Liu, Z., Zhang, Z. et al. SOCS2 inhibited mitochondria biogenesis via inhibiting p38 MAPK/ATF2 pathway in C2C12 cells. Mol Biol Rep 41, 627–637 (2014). https://doi.org/10.1007/s11033-013-2901-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2901-z