Abstract
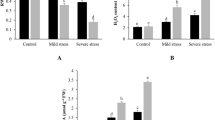
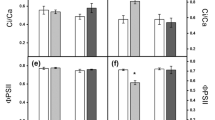
Abscisic acid is a plant hormone that participates in essential plant physiological processes, especially during adaptation to many environmental stresses, such as water deficit. The relationship between ABA accumulation and the expression of putative carotenoid cleavage dioxygenase (CCD) genes was investigated in the pot-cultivated leaves and roots of the ‘Rangpur’ lime and ‘Sunki Maravilha’ mandarin plants. Transpiration, stomatal resistance and leaf growth were evaluated when these genotypes were subjected to continuous water deficit. Under water deficit conditions, the ‘Rangpur’ lime extracts used greater amounts of water when compared to the ‘Sunki Maravilha’ plants, which reached the greatest stomatal resistance 5 days before ‘Rangpur’ lime. When subjected to water deficit, the roots and leaves of ‘Sunki Maravilha’ showed a progressive increase in ABA accumulation; however, in ‘Rangpur’ lime, alternations between high and low ABA concentrations were observed. These results suggest a retroactive feeding regulation by ABA. In ‘Rangpur’ lime the NCED2, NCED3 and CCD4a genes were expressed at the highest levels in the roots, and NCED5 was highly expressed in the leaves; in ‘Sunki Maravilha’, the NCED2 and NCED5 genes were most highly expressed in the roots, and NCED2 was most highly expressed in the leaves. However, for both genotypes, the transcription of these genes only correlated with ABA accumulation during the most severe water deficit conditions. The ‘Rangpur’ lime behaved as a vigorous rootstock; the leaf growth remained unaltered even when water was scarce. However, ‘Sunki Maravilha’ adaptation was based on the equilibrium of the response between the root and the aerial tissues due to water restriction. The use of the Sunki mandarin in combination with a scion with similar characteristics as its own, which responds to water deficit stress by accumulating ABA in the leaves, may display good drought tolerance under field conditions.







Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- FTSW:
-
Fraction of transpirable soil water
- NCED:
-
9-cis-Epoxycarotenoid dioxygenase
- NTR:
-
Normalised transpiration rate
- TDR:
-
Time domain reflectometry
References
Council I (2010) Climate change assessments review of the processes and procedures of the IPCC committee to review the intergovernmental panel on interacademy council interacademy council
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58:113–117
Rodríguez-Gamir J, Primo-Millo E, Forner JB et al (2010) Citrus rootstock responses to water stress. Sci Hortic 126:95–102
Moore JP, Vicré-Gibouin M, Farrant JM et al (2008) Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol Plant 134:237–245
Agusti J, Zapater M, Iglesias D et al (2007) Differential expression of putative 9-cis-epoxycarotenoid dioxygenases and abscisic acid accumulation in water stressed vegetative and reproductive tissues of citrus. Plant Sci 172:85–94
Hugouvieux V, Kwak JM, Schroeder JI (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106:477–487
Rodrigo M-J, Alquezar B, Zacarías L (2006) Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot 57:633–643
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7:41–48
Burbidge A, Grieve TM, Jackson A et al (1999) Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. Plant J 17:427–431
Qin X, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Natl Acad Sci USA 96:15354–15361
Chernys JT, Zeevaart JAD (2000) Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol 124:343–354
Neill SJ, Burnett EC, Desikan R et al (1998) Cloning of a wilt-responsive cDNA from an Arabidopsis thaliana suspension culture cDNA library that encodes a putative 9-cis-epoxy-carotenoid dioxygenase. J Exp Bot 49:1893–1894
Iuchi S, Kobayashi M, Taji T et al (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333
Soares Filho WDS, Cunha Sobrinho APD, Passos OS et al (2003) Maravilha: uma nova seleção de tangerina “Sunki”. Revista Brasileira de Fruticultura 25:268–271
Sinclair TR, Ludlow MM (1986) Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust J Plant Physiol 13:329–341
Kato M, Matsumoto H, Ikoma Y et al (2006) The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit. J Exp Bot 57:2153–2164
Malladi A, Burns JK (2008) CsPLDα1 and CsPLDγ1 are differentially induced during leaf and fruit abscission and diurnally regulated in Citrus sinensis. J Exp Bot 59:3729–3739
Bassene JB, Froelicher Y, Dhuique-Mayer CM et al (2009) Non-additive phenotypic and transcriptomic inheritance in a citrus allotetraploid somatic hybrid between C. reticulata and C. limon: the case of pulp carotenoid biosynthesis pathway. Plant Cell Rep 28:1689–1697
Irvine J, Perks MP, Magnani F et al (1998) The response of Pinus sylvestris to drought: stomatal control of transpiration and hydraulic conductance. Tree Physiol 18:393–402
Ray J, Sinclair TR (1998) The effect of pot size on growth and transpiration of maize and soybean during water deficit stress. J Exp Bot 49:1381–1386
Sadras VO, Milroy SP (1996) Soil-water thresholds for the responses of leaf expansion and gas exchange: a review. Field Crops Research 47:253–266
Morillon R, Chrispeels MJ (2001) The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Natl Acad Sci USA 98:14138–14143
Moya JL, Primo-Millo E, Talon M (1999) Morphological factors determining salt tolerance in citrus seedlings: the shoot to root ratio modulates passive root uptake of chloride ions and their accumulation in leaves. Plant Cell Environ 22:1425–1433
Moya J, Gómez-Cadenas A, Primo-Millo E et al (2003) Chloride absorption in salt-sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. J Exp Bot 54:825–833
Jorgensen S, Ntundu W, Ouédraogo M et al (2011) Effect of a short and severe intermittent drought on transpiration, seed yield, yield components, and harvest index in four landraces of bambara groundnut. Journal of Plant 5:25–36
Bright J, Desikan R, Hancock JT et al (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiolo 33:317–345
Sauter A, Davies WJ, Hartung W (2001) The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot 52:1991–1997
Schachtman DP, Goodger JQD (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. In: Kramer PJ, Boyer JS (eds) Water relations of plants and soil. Academic Press, The Netherlands, pp 201–256
Guerfel M, Beis A, Zotos T et al (2009) Differences in abscisic acid concentration in roots and leaves of two young Olive (Olea europaea L.) cultivars in response to water deficit. Acta Physiologiae Plantarum 31:825–831
Seo M, Koshiba T (2011) Transport of ABA from the site of biosynthesis to the site of action. J Plant Res 124:501–507
Cutler A, Krochko J (1999) Formation and breakdown of ABA. Trends Plant Sci 4:472–478
Qin X, Zeevaart JAD (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128:544–551
Seiler C, Harshavardhan VT, Rajesh K et al (2011) ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J Exp Bot 62:2615–2632
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Tan BC, Joseph LM, Deng WT et al (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Lefebvre V, North H, Frey A et al (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45:309–319
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci Drought 24:23–58
Neales TF, Masia A, Zhang J et al (1989) The effects of partially drying part of the root system of Helianthus annuus on the abscisic acid content of the roots, xylem sap and leaves. J Exp Bot 40:1113–1120
Stoll M, Loveys B, Dry P (2000) Hormonal changes induced by partial rootzone drying of irrigated grapevine. J Exp Bot 51:1627–1634
Ikegami K, Okamoto M, Seo M et al (2009) Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J Plant Res 122:235–243
Acknowledgments
The work of DMN was supported by the Coordination of Improvement of Higher Education Personnel (CAPES). This research was supported by Embrapa/Macroprograma II and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neves, D.M., Filho, M.A.C., Bellete, B.S. et al. Comparative study of putative 9-cis-epoxycarotenoid dioxygenase and abscisic acid accumulation in the responses of Sunki mandarin and Rangpur lime to water deficit. Mol Biol Rep 40, 5339–5349 (2013). https://doi.org/10.1007/s11033-013-2634-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2634-z