Abstract
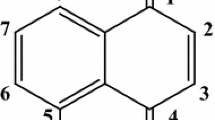
The interactions of artemisinins including artemisinin, dihydroartemisinin, artemether and artesunate with human serum albumin (HSA) were studied by fluorescence spectroscopy, UV–Vis absorption spectroscopy, synchronous fluorescence, three-dimensional fluorescence, circular dichroism (CD) and molecular modeling. Results obtained from analysis of fluorescence spectrum and fluorescence intensity indicated that the artemisinins had a strong ability to quench the intrinsic fluorescence of HSA through a static quenching procedure. Furthermore, the association constants K a and the corresponding thermodynamic parameters ΔH, ΔG and ΔS at various temperatures were also calculated. Based on the mechanism of Förster’s non-radiative energy transfer theory, the distance between the acceptors and HSA were found. In addition, alteration of the secondary structure of HSA in the presence of the artemisinins was tested by CD spectroscopy. Molecular modeling revealed that the artemisinins were bounded in the large hydrophobic cavity of the site I of HSA.









Similar content being viewed by others
References
Yang YZ, Asawamahasakda W, Meshnick SR (1993) Alkylation of human albumin by the antimalarial artemisinin. Biochem Pharmacol 46:336–339
Fishwick J, McLean WG, Edwards G, Ward SA (1995) The toxicity of artemisinin and related compounds on neuronal and glial cells in culture. Chem Biol Interact 96:263–271
Wesche DL, DeCoster MA, Tortella FC, Brewer TG (1994) Neurotoxicity of artemisinin analogs in vitro. Antimicrob Agents Chemother 38:1813–1819
Bian H, Li M, Yu Q, Chen Z, Tian J, Liang H (2006) Study of the interaction of artemisinin with bovine serum albumin. Int J Biol Macromol 39:291–297
Kragh-Hansen U (1981) Molecular aspects of ligand binding to serum albumin. Pharmacol Rev 33:17–53
Squella JA, Becerra R, Nunez-Vergara LJ (1987) Polarography: a new tool in the elucidation of drug-albumin interactions. Biochem Pharmacol 36:3531–3533
Peters T (1995) All about albumin: biochemistry, genetics and medical applications. Academic Press, San Diego, pp 1–40
Varshney A, Sen P, Ahmad E, Rehan M, Subbarao N, Khan RH (2010) Ligand binding strategies of human serum albumin: how can the cargo be utilized? Chirality 22:77–87
He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358:209–215
Petitpas I, Grüne T, Bhattacharya AA, Curry S (2001) Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol 314:955–960
Kragh-Hansen U (1990) Structure and ligand binding properties of human serum albumin. Dan Med Bull 37:57–84
Hervé F, Urien S, Albengres E (1994) Drug binding in plasma. A summary of recent tends in the study of drug and hormone binding. Clin Pharmacokinet 26:44–58
Bogdan M, Pimau A, Floare C (2008) Binding interaction of indomethacin with human serum albumin. J Pharm Biomed Anal 47:981–984
Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK (2006) Interactions of antitumor metallodrugs with serum proteins: advances in characterization using modern analytical methodology. Chem Rev 106:2224–2248
Zhang GJ, Keita B, Craescu CT, Miron S, de Oliveira P, Nadjo L (2008) Molecular interactions between Wells-Dawson type polyoxometalates and human serum albumin. Biomacromolecules 9:812–817
Gentili PL, Ortica F, Favaro G (2008) Static and dynamic interaction of a naturally occurring photochromic molecule with bovine serum albumin studied by UV–Visible absorption and fluorescence spectroscopy. J Phys Chem B 112:16793–16801
Wang F, Huang W, Dai ZX (2008) Spectroscopic investigation of the interaction between riboflavin and bovine serum albumin. J Mol Struct 875:509–514
Chakraborty B, Basu S (2009) Interaction of BSA with proflavin: a spectroscopic approach. J Lumin 129:34–39
Ferrer EG, Bosch A, Yantorno O, Baran EJ (2008) A spectroscopy approach for the study of the interactions of bioactive vanadium species with bovine serum albumin. Bioorg Med Chem 16:3878–3886
Kathiravan A, Chandramohan M, Renganathan R, Sekar S (2009) Spectroscopic studies on the interaction between phycocyanin and bovine serum albumin. J Mol Struct 919:210–214
Kalanur SS, Seetharamappa J, Kalalbandi VK (2010) Characterization of interaction and the effect of carbamazepine on the structure of human serum albumin. J Pharm Biomed Anal 53:660–666
Matei I, Hillebrand M (2010) Interaction of kaempferol with human serum albumin: a fluorescence and circular dichroism study. J Pharm Biomed Anal 51:768–773
Fotouhi L, Banafsheh S, Heravi MM (2009) Electrochemistry of the interaction of furazolidone and bovine serum albumin. Bioelectrochemistry 77:26–30
Równicka-Zubik J, Sulkowska A, Pożycka J, Gaździcka K, Bojko B, Maciażek-Jurczyk M, Sulkowski WW (2009) Fluorescence analysis of sulfasalazine bound to defatted serum albumin in the presence of denaturation factors. J Mol Struct 924–26:371–377
Channu BC, Kalpana HN, Eregowda GB, Dass C, Houghton PJ, Thimmaiah KN (1999) Interaction of substituted phenoxazine chemosensitizers with bovine serum albumin. J Pharm Biomed Anal 21:775–785
Hu YJ, Liu Y, Zhang LX, Zhao RM, Qu SS (2005) Studies of interaction between colchicine and bovine serum albumin by fluorescence quenching method. J Mol Struct 750:174–178
Silva D, Cortez CM, Cunha-Bastos J, Louro SR (2004) Methyl parathion interaction with human and bovine serum albumin. Toxicol Lett 147:53–61
Petitpas I, Bhattacharya AA, Twine S, East M, Curry S (2001) Crystal structure analysis of warfarin binding to human serum albumin: anatomy of drug site I. J Biol Chem 276:22804–22809
Morris G (2002) SYBYL Software, Version 6.9. Louis, St., Tripos Associates
Lakowicz JR (2008) Principles of fluorescence spectroscopy, 3rd edn. Plenum Press, New York, p 277
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Plenum Press, New York, pp 237–265
Ahmad B, Khan RH (2006) Studies on the acid unfolded and molten globule states of catalytically active stem bromelain: a comparison with catalytically inactive form. J Biochem 140:501–508
Hu YJ, Liu Y, Zhao RM, Dong JX, Qu SS (2006) Spectroscopic studies on the interaction between methylene blue and bovine serum albumin. J Photoch Photobio A 179:324–329
Wang J, Zhang YY, Guo Y, Zhang L, Xu R, Xing ZQ, Wang SX, Zhang XD (2009) Interaction of bovine serum albumin with acridine orange (C.I. basic orange 14) and its sonodynamic damage under ultrasonic irradiation. Dyes Pigment 80:271–278
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry 12:4161–4170
Zhang YZ, Zhou B, Liu YX, Zhou CX, Ding XL, Liu Y (2008) Fluorescence study on the interaction of bovine serum albumin with p-aminoazobenzene. J Fluoresc 18:109–118
Yang MM, Qin X, Xi XL (2006) Study of the interaction of cephalosporin class medicine with albumin by fluorescence enhancement and fluorescence quenching theories. Chin J Chem 24:642–648
Zhao H, Ge M, Zhang Z, Wang W, Wu G (2006) Spectroscopic studies on the interaction between riboflavin and albumins. Spectrochim Acta Mol Biomol Spectrosc 65:811–817
Leckband D (2000) Measuring the forces that control protein interactions. Annu Rev Biophys Biomol Struct 29:1–26
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20:3096–3102
Förster T (1948) Energy transfer and fluorescence between molecules. Ann Phys 437:55–75
Mahammed A, Gray HB, Weaver JJ, Sorasaenee K, Gross Z (2004) Amphiphilic corroles bind tightly to human serum albumin. Bioconjug Chem 15:738–746
Cyril L, Earl JK, Sperry WM (1961) Biochemists’ handbook. E & FN Spon, London, p 84
Valeur B, Brochom JC (2001) New trends in fluorescence spectroscopy. Springer, Berlin, p 25
Alexander V, Pastukhov V, Levchenko LA, Sadkov AP (2007) Spectroscopic study on binding of rutin to human serum albumin. J Mol Struct 842:60–66
Yang P, Gao F (2002) The principle of bioinorganic chemistry. Science Press, Beijing, p 349
Kamat BP, Seetharamappa J (2004) In vitro study on the interaction of mechanism of tricyclic compounds with bovine serum albumin. J Pharm Biomed Anal 35:655–664
Lu ZX, Cui T, Shi QL (1987) Applications of circular dichroism (CD) and optical rotatory dispersion (ORD) in molecular biology, 1st edn. Science Press, Beijing
Hu YJ, Liu Y, Wang JB, Xiao XH, Qu SS (2004) Study of the interaction between monoammonium glycyrrhizinate and bovine serum albumin. J Pharm Biomed Anal 36:915–919
Congdon RW, Muth GW, Splittqerber AG (1993) The binding interaction of coomassie blue with proteins. Anal Biochem 213:407–413
Wang YQ, Tang BP, Zhang HM, Zhou QH, Zhang GC (2009) Studies on the interaction between imidacloprid and human serum albumin: spectroscopic approach. J Photochem Photobiol B 94:183–190
Zhang YZ, Dai J, Zhang XP, Yang X, Liu Y (2008) Studies of the interaction between Sudan I and bovine serum albumin by spectroscopic methods. J Mol Struct 888:152–159
Tian J, Liu J, Hu Z, Chen X (2005) Interaction of wogonin with bovine serum albumin. Bioorg Med Chem 13:4124–4129
Bojesen IN, Hansen HS (2003) Binding of anandamide to bovine serum albumin. J Lipid Res 44:1790–1794
Hou HN, Qi ZD, Ouyang YW, Liao FL, Zhang Y, Liu Y (2008) Studies on interaction between Vitamin B12 and human serum albumin. J Pharm Biomed Anal 47:134–139
Acknowledgments
We gratefully acknowledge our institute to provide the Fluorescence Spectrofluorimeter and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Jiang, H. & Pu, H. Interaction of artemisinin and its derivatives with human serum albumin studied using spectroscopies and molecular modeling methods. Mol Biol Rep 40, 4791–4804 (2013). https://doi.org/10.1007/s11033-013-2575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2575-6