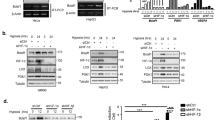
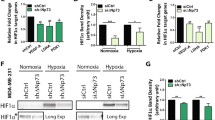
Abstract
Intratumoral hypoxia is associated with poor prognosis, regardless of the mode of therapy. Cancer cells survive this condition through activating several adaptive signaling pathways, including the integrated stress response (ISR) and autophagy. Activating transcription factor 4 (ATF4) is the major transcriptional mediator of the ISR, which we have shown to be involved in autophagy regulation to protect cells from severe hypoxia. Here we demonstrate that ATF4 orchestrates a program of BH3-only protein expression in severe hypoxia. We find that the BH3-only proteins HRK, PUMA, and NOXA are transcriptionally induced in severe hypoxia and that their expression is abrogated by RNA interference against ATF4. In particular, we show that the BH3-only protein harakiri (HRK) is transactivated by ATF4 in severe hypoxia through direct binding of ATF4 to the promoter region. Furthermore, we demonstrate through siRNA knockdown that HRK induces autophagy and promotes cancer cell survival in severe hypoxia.






Similar content being viewed by others
References
Thomlinson RH, Gray LH (1955) The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 9:539–549
Bertout JA, Patel SA, Simon MC (2008) The impact of O2 availability on human cancer. Nat Rev Cancer 8:967–975
Wouters BG, Koritzinsky M (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 8:851–864
Hockel M, Vorndran B, Schlenger K, Baussmann E, Knapstein PG (1993) Tumor oxygenation: a new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol Oncol 51:141–149
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ et al (1996) Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379:88–91
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2:38–47
Tu BP, Weissman JS (2002) The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell 10:983–994
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD et al (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633
Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K et al (2006) Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J 25:1114–1125
Smirnova JB, Selley JN, Sanchez-Cabo F, Carroll K, Eddy AA et al (2005) Global gene expression profiling reveals widespread yet distinctive translational responses to different eukaryotic translation initiation factor 2B-targeting stress pathways. Mol Cell Biol 25:9340–9349
Blais JD, Filipenko V, Bi M, Harding HP, Ron D et al (2004) Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol 24:7469–7482
Ameri K, Harris AL (2008) Activating transcription factor 4. Int J Biochem Cell Biol 40:14–21
Fels DR, Koumenis C (2006) The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther 5:723–728
Ma Y, Hendershot LM (2004) The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 4:966–977
Heath-Engel HM, Chang NC, Shore GC (2008) The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene 27:6419–6433
Mizushima N (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22:132–139
Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL (2001) HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61:6669–6673
Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P et al (2007) BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 27:6229–6242
Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J et al (2009) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 120:127–141
Rzymski T, Milani M, Pike L, Buffa F, Mellor HR et al (2010) Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene 29:4424–4435
Milani M, Rzymski T, Mellor HR, Pike L, Bottini A et al (2009) The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res 69:4415–4423
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B et al (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676
Elgendy M, Sheridan C, Brumatti G, Martin SJ (2011) Oncogenic ras-induced expression of noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell 42(1):23–35
Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F et al (2007) Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26:2527–2539
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH et al (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122:927–939
Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH (2009) PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ 16:1135–1145
Coultas L, Terzano S, Thomas T, Voss A, Reid K et al (2007) Hrk/DP5 contributes to the apoptosis of select neuronal populations but is dispensable for haematopoietic cell apoptosis. J Cell Sci 120:2044–2052
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G et al (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4:151–175
Rzymski T, Harris AL (2007) The unfolded protein response and integrated stress response to anoxia. Clin Cancer Res 13:2537–2540
Mujcic H, Rzymski T, Rouschop KM, Koritzinsky M, Milani M et al (2009) Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol 92:450–459
Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K et al (2004) Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev 18:2095–2107
Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH (2004) BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med 199:113–124
Ishihara T, Hoshino T, Namba T, Tanaka K, Mizushima T (2007) Involvement of up-regulation of PUMA in non-steroidal anti-inflammatory drug-induced apoptosis. Biochem Biophys Res Commun 356:711–717
Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS et al (2010) Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci 30:16938–16948
Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C et al (2009) ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA 106:2200–2205
Rzymski T, Milani M, Singleton DC, Harris AL (2009) Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle 8:3838–3847
Inohara N, Ding L, Chen S, Nunez G (1997) harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J 16:1686–1694
Wakabayashi T, Kosaka J, Hommura S (2002) Up-regulation of Hrk, a regulator of cell death, in retinal ganglion cells of axotomized rat retina. Neurosci Lett 318:77–80
Gurzov EN, Ortis F, Cunha DA, Gosset G, Li M et al (2009) Signaling by IL-1beta + IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ 16:1539–1550
Young JE, Garden GA, Martinez RA, Tanaka F, Sandoval CM et al (2009) Polyglutamine-expanded androgen receptor truncation fragments activate a Bax-dependent apoptotic cascade mediated by DP5/Hrk. J Neurosci 29:1987–1997
Imaizumi K, Morihara T, Mori Y, Katayama T, Tsuda M et al (1999) The cell death-promoting gene DP5, which interacts with the BCL2 family, is induced during neuronal apoptosis following exposure to amyloid beta protein. J Biol Chem 274:7975–7981
Aoki S, Su Q, Li H, Nishikawa K, Ayukawa K et al (2002) Identification of an axotomy-induced glycosylated protein, AIGP1, possibly involved in cell death triggered by endoplasmic reticulum-Golgi stress. J Neurosci 22:10751–10760
Duennwald ML, Lindquist S (2008) Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev 22:3308–3319
Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P et al (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 174:1241–1251
Lee do Y, Lee KS, Lee HJ, Kim do H, Noh YH et al (2010) Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS ONE 5:e10489
Shimoke K, Sasaya H, Ikeuchi T (2011) Analysis of the role of nerve growth factor in promoting cell survival during endoplasmic reticulum stress in PC12 cells. Methods Enzymol 490:53–70
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Towers E, Gilley J, Randall R, Hughes R, Kristiansen M et al (2009) The proapoptotic dp5 gene is a direct target of the MLK-JNK-c-Jun pathway in sympathetic neurons. Nucleic Acids Res 37:3044–3060
van den Beucken T, Koritzinsky M, Niessen H, Dubois L, Savelkouls K et al (2009) Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. J Biol Chem 284:24204–24212
Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF (2005) The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem 280:20331–20339
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ et al (2005) Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 25:5675–5686
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH et al (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283:10892–10903
Mortensen M, Watson AS, Simon AK (2011) Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy 7:1069–1070
Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M et al (2011) The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 208:455–467
Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D et al (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10:51–64
Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R et al (2007) Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev 21:1621–1635
Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K et al (2007) Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 21:1367–1381
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G et al (2009) Autophagy suppresses tumorigenesis through elimination of p62. Cell 137:1062–1075
Kroemer G, Levine B (2008) Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9:1004–1010
Lenardo MJ, McPhee CK, Yu L (2009) Autophagic cell death. Methods Enzymol 453:17–31
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293
Yang S, Wang X, Contino G, Liesa M, Sahin E et al (2011) Pancreatic cancers require autophagy for tumor growth. Genes Dev 25:717–729
Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM et al (2011) Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 25:460–470
Nakamura M, Ishida E, Shimada K, Nakase H, Sakaki T et al (2005) Frequent HRK inactivation associated with low apoptotic index in secondary glioblastomas. Acta Neuropathol 110:402–410
Nakamura M, Shimada K, Konishi N (2008) The role of HRK gene in human cancer. Oncogene 27(Suppl 1):S105–S113
Obata T, Toyota M, Satoh A, Sasaki Y, Ogi K et al (2003) Identification of HRK as a target of epigenetic inactivation in colorectal and gastric cancer. Clin Cancer Res 9:6410–6418
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7:673–682
Li J, Lee B, Lee AS (2006) Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem 281:7260–7270
Rzymski T, Paantjens A, Bod J, Harris AL (2008) Multiple pathways are involved in the anoxia response of SKIP3 including HuR-regulated RNA stability, NF-kappaB and ATF4. Oncogene 27:4532–4543
Otsuki Y, Li Z, Shibata MA (2003) Apoptotic detection methods–from morphology to gene. Prog Histochem Cytochem 38:275–339
Acknowledgments
Funding from Cancer Research UK, the Rhodes Trust, and the Natural Sciences and Engineering Research Council of Canada supported this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare no competing interests, financial or otherwise.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pike, L.R.G., Phadwal, K., Simon, A.K. et al. ATF4 orchestrates a program of BH3-only protein expression in severe hypoxia. Mol Biol Rep 39, 10811–10822 (2012). https://doi.org/10.1007/s11033-012-1975-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1975-3