Abstract
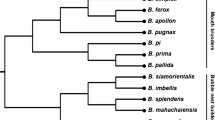
Myostatin (MSTN) is a member of the transforming growth factor-β superfamily and functions as a negative regulator of skeletal muscle development and growth. In this study, the bighead carp MSTN gene (AnMSTN for short) was cloned and characterized. The 3,769 bp genomic sequence of AnMSTN consisted of three exons and two introns, and the full length cDNA (2,141 bp) of the gene had an open reading frame encoding a polypeptide of 375 amino acids. The deduced amino acid sequence of AnMSTN showed 67.1–98.7 % homology with MSTNs of avian, mammalian and teleostean species. Sequence comparison and phylogenetic analysis confirmed the MSTNs were conserved throughout the vertebrates and AnMSTN belonged to MSNT-1 isoform. AnMSTN was expressed in various tissues with the highest expression in muscle. Two single nucleotide polymorphisms, g.1668T > C in intron 2 and g.2770C > A in 3′ UTR, were identified in AnMSTN by sequencing PCR fragments, and genotyped by SSCP. Association analysis showed that g.2770C > A genotypes were significantly associated with total length, body length and body weight (P < 0.01). These results suggest that AnMSTN involves in the regulation of growth, and this polymorphism would be informative for further studies on selective breeding in bighead carp.





Similar content being viewed by others
References
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M (1997) A deletion in the bovine myostatin gene causes the double muscled phenotype in cattle. Nat Genet 17:71–74
Lee CY, Hu SY, Gong HY, Chen MH, Lu JK, Wu JL (2009) Suppression of myostatin with vector-based RNA interference causes a double-muscle effect in transgenic zebrafish. Biochem Biophys Res Commun 387:766–771
Sawatari E, Seki R, Adachi T, Hashimoto H, Uji S, Wakamatsu Y, Nakata T, Kinoshita M (2010) Overexpression of the dominant-negative form of myostatin results in doubling of muscle-fiber number in transgenic medaka (Oryzias latipes). Comp Biochem Physiol A 155:183–189
Rodgers BD, Weber GM, Sullivan CV, Evine MA (2001) Isolation and characterization of myostatin complementary deoxyribonucleic acid clones from two commercially important fish: Oreochromis mossambicus and Morone chrysops. Endocrinology 142:1412–1418
Xue LY, Qian KX, Qian HQ, Li L, Yang QY, Li MY (2006) Molecular cloning and characterization of the myostatin gene in croceine croaker, Pseudosciaena crocea. Mol Biol Rep 33:129–135
Ko CF, Chiou TT, Chen T, Wu JL, Chen JC, Lu JK (2007) Molecular cloning of myostatin gene and characterization of tissue-specific and developmental stage-specific expression of the gene in orange spotted grouper, Epinephelus coioides. Mar Biotechnol 9:20–32
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311
Østbye TK, Galloway TF, Nielsen C, Gabestad I, Bardal T, Andersen Ø (2001) The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur J Biochem 268:5249–5257
Biga PR, Roberts SB, Iliev DB, McCauley LA, Moon JS, Collodi P, Goetz FW (2005) The isolation, characterization, and expression of a novel GDF11 gene and a second myostatin form in zebrafish, Danio rerio. Comp Biochem Physiol B 141:218–230
De Santis C, Jerry DR (2011) Differential tissue-regulation of myostatin genes in the teleost fish Lates calcarifer in response to fasting. Evidence for functional differentiation. Mol Cell Endocrinol 335:158–165
Maccatrozzo L, Bargelloni L, Cardazzo B, Rizzo G, Patarnello T (2001) A novel second myostatin gene is present in teleost fish. FEBS Lett 509:36–40
Helterline DL, Garikipati D, Stenkamp DL, Rodgers BD (2007) Embryonic and tissue-specific regulation of myostatin-1 and 2 gene expression in zebrafish. Gen Comp Endocrinol 151:90–97
Lynch M, Walsh B (1997) Genetics and analysis of quantitative traits. Sinauer Associates, Inc., Sunderland, p 980
Jiang YL, Li N, Fan XZ, Xiao LR, Xiang RL, Hu XX, Du LX, Wu CX (2002) Associations of T → A mutation in the promoter region of myostatin gene with birth weight in Yorkshire pigs. AJAS 15:154–1545
Sellick GS, Pitchford WS, Morris CA, Cullen NG, Crawford AM, Raadsma HW, Bottema CD (2007) Effect of myostatin F94L on carcass yield in cattle. Anim Genet 38:440–446
Hickford JG, Forrest RH, Zhou H, Fang Q, Han J, Frampton CM, Horrell AL (2010) Polymorphisms in the ovine myostatin gene (MSTN) and their association with growth and carcass traits in New Zealand Romney sheep. Anim Genet 41:64–72
Wang XL, Meng XY, Song B, Qiu XM, Liu HY (2010) SNPs in the myostatin gene of the mollusk Chlamys farreri: association with growth traits. Comp Biochem Physiol B 155:327–330
Tang YK, Li JL, Yu JH, Chen XF, Li HX (2010) Genetic structure of MSTN and association between its polymorphisms and growth traits in genetically improved farmed tilapia (GIFT). J Fish Sci China 17:44–51
FAO (Food and Agriculture Organization) (2010) Fishery and aquaculture statistics 2008. Food and Agriculture Organization of the United Nations, Rome
Hu PP, Datto MB, Wang XG (1998) Molecular mechanisms of transforming growth factor-beta signaling. Endocr Rev 19:349–363
Daopin S, Piez KA, Ogawa Y, Davies DR (1992) Crystal structure of transforming growth factor-beta2: an unusual fold for the superfamily. Science 257:369–373
Kerr T, Roalson EH, Rodgers BD (2005) Phylogenetic analysis of the myostatin gene sub-family and the differential expression of a novel member in zebrafish. Evol Dev 7:390–400
Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282:1711–1714
Postlethwait JH, Yan YL, Gates MA, Horne S, Amores A, Brownlie A, Donovan A, Egan ES, Force A, Gong Z, Goutel C, Fritz A, Kelsh R, Knapik E, Liao E, Paw B, Ransom D, Singer A, Thomson M, Abduljabbar TS, Yelick P, Beier D, Joly JS, Larhammar D, Rosa F, Westerfield M, Zon LI, Johnson SL, Talbot WS (1998) Vertebrate genome evolution and the zebrafish gene map. Nat Genet 18:345–349
Turner BJ (1984) Evolutionary genetics of fishes. Plenum Press, New York
David L, Blum S, Feldman MW, Lavi U, Hillel J (2003) Recent duplication of the common carp (Cyprinus carpio L.) genome as revealed by analyses of microsatellite loci. Mol Biol Evol 20:1425–1434
Ji S, Losinski RL, Cornellius SG, Frank GR, Willi GM, Gerrard EE, Depreux FF, Spurlock ME (1998) Myostatin expression in porcine tissues: tissue specificity and developmental and postnatal regulation. Am J Physiol 275:1265–1273
Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ (1999) Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol 180:1–9
De Santis C, Evans BS, Smith-Keune C, Jerry DR (2008) Molecular characterization, tissue expression and sequence variability of the barramundi (Lates calcarifer) myostatin gene. BMC Genomics 9:82–96
Kocabas AM, Kucuktas H, Dunham RA, Liu ZJ (2002) Molecular characterization and differential expression of the myostatin gene in channel catfish (Ictalurus punctatus). Biochim Biophys Acta 1575:99–107
Hadjipavlou G, Matika O, Clop A, Bishop SC (2008) Two single nucleotide polymorphisms in the myostatin (GDF8) gene have significant association with muscle depth of commercial Charollais sheep. Anim Genet 39:346–353
Yu L, Tang H, Wang J, Wu Y, Zou L, Jiang Y, Wu C, Li N (2007) Polymorphisms in the 5′ regulatory region of myostatin gene are associated with early growth traits in Yorkshire pigs. Sci China C Life Sci 50:642–647
Yu JH, Li HX, Tang YK, Li JL, Dong ZJ (2010) Isolation and expression of myostatin (MSTN) genes, and their polymorphism correlations with body form and average daily gain in Cyprinus carpio var. jian. Chin J Agric Biotechnol 18:1062–1072
Glazier AM, Nadeau JH, Aitman TJ (2002) Finding genes that underlie complex traits. Science 298:2345–2349
Pagani F, Baralle FE (2004) Genomic variants in exons and introns: identifying the splicing spoilers. Nat Rev Genet 5:389–396
Stanilova S, Miteva L, Prakova G (2007) Interleukin-12B-3′ UTR polymorphism in association with IL-12p40 and IL-12p70 serum levels and silicosis severity. Int J Immunogenet 34:193–199
Pickard B, Knight HM, Hamilton RS, Soares DC, Walker R, Boyd JKF, Machell J, Maclean A, McGhee KA, Condie A, Porteous DJ, St Clair D, Davis I, Blackwood DHR, Muir WJA (2008) Common variant in the 3′ UTR of the GRIK4 glutamate receptor gene affects transcript abundance and protects against bipolar disorder. Proc Natl Acad Sci USA 105:14940–14945
Liu LQ, Li FE, Deng CY, Xiong YZ (2011) Molecular cloning, tissue expression and association of porcine NR4A1 gene with reproductive traits. Mol Biol Rep 38:103–114
Liu CX, Gao H, Zhai SL, Liu B (2011) Molecular characterization, chromosomal localization, expression profile and association analysis with carcass traits of the porcine dickkopf homolog1 gene. Mol Biol Rep 38:1929–1934
Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibé B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, Laville E, Meish F, Milenkovic D, Tobin J, Charlier C, Georges M (2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38:813–818
Antonarakis SE, Cooper DN (2003) Mutations in Human Genetic Diseases: nature and consequences. Nature Encyclopedia of the Human Genome. Nature Publishing Group, London, 4:227–253
Pesole G, Mignone F, Gissi C, Grillo G, Licciulli F, Liuni S (2001) Structural and functional features of eukaryotic mRNA untranslated regions. Gene 276:73–81
Fu L, Benchimol S (1997) Participation of the human p53 3′ UTR in translational repression and activation following γ-irradiation. EMBO J 16:4117–4725
Maquat LE (2001) The power of point mutations. Nature Genet 27:5–6
Kuhnlein U, Ni L, Weigend S, Gavora JS, Fairfull W, Zadworny D (1997) DNA polymorphisms in the chicken growth hormone gene: response to selection for disease resistance and association with egg production. Anim Genet 28:116–123
Hayes B, Baranski M, Goddard ME, Robinson N (2007) Optimisation of marker assisted selection for abalone breeding programs. Aquaculture 265:61–69
Casas E, Shackelford SD, Keele JW, Stone RT, Kappers SM, Koohmaraie M (2000) Quantitative trait loci affecting growth and carcass composition of cattle segregating alternative from of myostatin. J Anim Sci 78:560–569
Marcq F, Elsen JM, El Barkouki S, Bouix J, Eychenne F, Grobet L, Karim L, Laville E, Nezer C, Royo L, Sayd T, Bibé B, Le Roy PL, Georges M (1998) Investigating the role of double muscling characterizing Belgian Texel sheep. Anim Genet 29(supp.1):52–53
Acknowledgments
This study was funded by the Ministry of Agriculture of China (200903045, 2011-G12). Parts of the data used were generated within the project MOST 973 (2010CB126305) and FEBL (2011FBZ20). L. L was financially supported by CAS studentship. We thank Mr. C. Zhu and W. Guo, F. Zeng for the collection of fish samples and technical assistance in laboratory analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2012_1794_MOESM2_ESM.tif
Fig. S1 Nucleotide sequence and deduced amino acid sequence for the AnMSTN. Exons are shown in capital letters and introns in small letters. Nucleotides are numbered from the first base at the 5′ end. (TIFF 3180 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Yu, X. & Tong, J. Molecular characterization of myostatin (MSTN) gene and association analysis with growth traits in the bighead carp (Aristichthys nobilis). Mol Biol Rep 39, 9211–9221 (2012). https://doi.org/10.1007/s11033-012-1794-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1794-6