Abstract
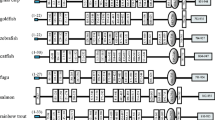
Toll-like receptors (TLRs) play a crucial role in host defence, since they trigger immune response following recognition of pathogen-associated molecular patterns (PAMPs) in potential infectious agents. TLRs have been found in numerous organisms, including mammals, birds and teleosts. Some TLR members are commonly retained across all species, whilst others were lost, gained or diverged independently during evolution. Our knowledge about the evolution and specific functions of tlr21, tlr22 and tlr23 in teleosts are still scarce. Phylogenetic analysis of 18 tlr13, tlr21, tlr22 and tlr23 genes from 9 different fish species divided them in two groups. All tlr21 genes were under the first clade, while the second comprised tlr22, tlr23 and tlr13 from Atlantic salmon. Evidence of positive selection was detected at three sites within the leucine-rich repeat regions of Tlr22, which may influence PAMP recognition. Immunostimulation experiments revealed that expression of zebrafish tlr22 is modulated by several unrelated PAMPs. Up to a 3-fold increase in tlr21 and tlr22 expression was detected in larvae exposed to immunostimulants such as lipopolysaccharide, peptidoglycan or poly I:C. We found that zebrafish tlrs are expressed mainly in immune-related organs, such as spleen and kidney as well as in testis and temperature stress did not have an effect on the expression of tlr21 and tlr22 in the early stages of development in zebrafish larvae. Our data indicates that these teleost tlrs may play a role in innate host defence. In particular, tlr22 is evolving under positive selection, which indicates functional diversification and adaptation of the response to different PAMPs.






Similar content being viewed by others
Abbreviations
- Tlr:
-
Toll-like receptor
- PAMPs:
-
Pathogen-associated molecular patterns
- LRR:
-
Leucine-rich repeat
- LPS:
-
Lipopolysaccharide from Salmonella enterica typhimurium
- IC:
-
Polyinosinic-polycytidylic acid potassium salt
- PG:
-
Peptidoglycan from Staphylococcus aureus
References
Smith VJ, Fernandes JMO (2009) Antimicrobial peptides of the innate immune system. In: Zaccone G, Perriere C, Mathis A, Kapoor BG (eds) Fish defences: pathogens, parasites and predators. Science Publishers, USA, pp 241–276. doi:10.1201/b10188-9
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216. doi:10.1146/annurev.immunol.20.083001.084359
Medzhitov R, Janeway CA Jr (1998) Innate immune recognition and control of adaptive immune responses. Semin Immunol 10(5):351–353. doi:10.1006/smim.1998.0136
Kumar H, Kawai T, Akira S (2009) Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388(4):621–625. doi:10.1016/j.bbrc.2009.08.062
Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449(7164):819–826. doi:10.1038/nature06246
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801. doi:10.1016/j.cell.2006.02.015
Jin MS, Lee JO (2008) Structures of the toll-like receptor family and its ligand complexes. Immunity 29(2):182–191. doi:10.1016/j.immuni.2008.07.007
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11(5):373–384. doi:10.1038/ni.1863
Hashimoto C, Hudson KL, Anderson KV (1988) The Toll gene of drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52(2):269–279. doi:10.1016/0092-8674(88)90516-8
Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86(6):973–983. doi:10.1016/S0092-8674(00)80172-5
Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A (2005) The evolution of vertebrate toll-like receptors. Proc Natl Acad Sci USA 102(27):9577–9582. doi:10.1073/pnas.0502272102
Brownlie R, Allan B (2011) Avian toll-like receptors. Cell Tissue Res 343(1):121–130. doi:10.1007/s00441-010-1026-0
Rebl A, Goldammer T, Seyfert HM (2010) Toll-like receptor signaling in bony fish. Vet Immunol Immunopathol 134(3–4):139–150. doi:10.1016/j.vetimm.2009.09.021
Wlasiuk G, Nachman MW (2010) Adaptation and constraint at toll-like receptors in primates. Mol Biol Evol 27(9):2172–2186. doi:10.1093/molbev/msq104
Cormican P, Lloyd AT, Downing T, Connell SJ, Bradley D, O’Farrelly C (2009) The avian Toll-Like receptor pathway-subtle differences amidst general conformity. Dev Comp Immunol 33(9):967–973. doi:10.1016/j.dci.2009.04.001
Chen JS, Wang TY, Tzeng TD, Wang CY, Wang D (2008) Evidence for positive selection in the TLR9 gene of teleosts. Fish Shellfish Immunol 24(2):234–242. doi:10.1016/j.fsi.2007.11.005
Novoa B, Figueras A (2012) Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv Exp Med Biol 946:253–275. doi:10.1007/978-1-4614-0106-3_15
Guindon S, Delsuc F, Dufayard JF, Gascuel O (2009) Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol 537:113–137. doi:10.1007/978-1-59745-251-9_6
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. doi:10.1093/bioinformatics/btg180
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24(8):1586–1591. doi:10.1093/molbev/msm088
Fernandes JM, Ruangsri J, Kiron V (2010) Atlantic cod piscidin and its diversification through positive selection. PLoS One 5(3):e9501. doi:10.1371/journal.pone.0009501
Consuegra S, Megens HJ, Leon K, Stet RJM, Jordan WC (2005) Patterns of variability at the major histocompatibility class II alpha locus in Atlantic salmon contrast with those at the class I locus. Immunogenetics 57(1):16–24. doi:10.1007/s00251-004-0765-z
Sarropoulou E, Fernandes JMO, Mitter K, Magoulas A, Mulero V, Sepulcre MP, Figueras A, Novoa B, Kotoulas G (2010) Evolution of a multifunctional gene: the warm temperature acclimation protein Wap65 in the European seabass Dicentrarchus labrax. Mol Phylogenet Evol 55(2):640–649. doi:10.1016/j.ympev.2009.10.001
Delport W, Poon AFY, Frost SDW, Kosakovsky Pond SL (2010) Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26(19):2455–2457. doi:10.1093/bioinformatics/btq429
Campos C, Valente LMP, Fernandes JMO (2012) Molecular evolution of zebrafish dnmt3 genes and thermal plasticity of their expression during embryonic development. Gene 500(1):93–100. doi:10.1016/j.gene.2012.03.041
Novoa B, Bowman TV, Zon L, Figueras A (2009) LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish Immunol 26(2):326–331. doi:10.1016/j.fsi.2008.12.004
Johansen A, Collet B, Sandaker E, Secombes CJ, Jørgensen JB (2004) Quantification of Atlantic salmon type-I interferon using an Mx1 promoter reporter gene assay. Fish Shellfish Immunol 16(2):173–184. doi:10.1016/s1050-4648(03)00060-3
Campos C, Valente LMP, Borges P, Bizuayehu T, Fernandes JMO (2010) Dietary lipid levels have a remarkable impact on the expression of growth-related genes in Senegalese sole (Solea senegalensis Kaup). J Exp Biol 213(2):200–209. doi:10.1242/jeb.033126
Wheelan SJ, Church DM, Ostell JM (2001) Spidey: a tool for mRNA-to-genomic alignments. Genome Res 11(11):1952–1957. doi:10.1101/gr.195301
Fernandes JM, Mommens M, Hagen O, Babiak I, Solberg C (2008) Selection of suitable reference genes for real-time PCR studies of Atlantic halibut development. Comp Biochem Physiol B Biochem Mol Biol 150(1):23–32. doi:10.1016/j.cbpb.2008.01.003
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36. doi:10.1093/nar/30.9.e36
Kobe B, Kajava AV (2001) The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11(6):725–732. doi:10.1016/s0959-440x(01)00266-4
Matsushima N, Tanaka T, Enkhbayar P, Mikami T, Taga M, Yamada K, Kuroki Y (2007) Comparative sequence analysis of leucine-rich repeats (LRRs) within vertebrate toll-like receptors. BMC Genomics 8:124–143. doi:10.1186/1471-2164-8-124
Bell JK, Mullen GED, Leifer CA, Mazzoni A, Davies DR, Segal DM (2003) Leucine-rich repeats and pathogen recognition in toll-like receptors. Trends Immunol 24(10):528–533. doi:10.1016/s1471-4906(03)00242-4
Bell JK, Botos I, Hall PR, Askins J, Shiloach J, Segal DM, Davies DR (2005) The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc Natl Acad Sci USA 102(31):10976–10980. doi:10.1073/pnas.0505077102
Palti Y (2011) Toll-like receptors in bony fish: from genomics to function. Dev Comp Immunol 35(12):1263–1272. doi:10.1016/j.dci.2011.03.006
Leong J, Jantzen S, von Schalburg K, Cooper G, Messmer A, Liao N, Munro S, Moore R, Holt R, Jones S, Davidson W, Koop B (2010) Salmo salar and Esox lucius full-length cDNA sequences reveal changes in evolutionary pressures on a post-tetraploidization genome. BMC Genomics 11(1):279–295. doi:10.1186/1471-2164-11-279
Yang Z, Wong WS, Nielsen R (2005) Bayes empirical Bayes inference of amino acid sites under positive selection. Mol Biol Evol 22(4):1107–1118. doi:10.1093/molbev/msi097
Park S, Park D, Jung Y-J, Chung E, Choi S (2010) Positive selection signatures in the TLR7 family. Genes Genomics 32(2):143–150. doi:10.1007/s13258-009-0837-4
Areal H, Abrantes J, Esteves P (2011) Signatures of positive selection in Toll-like receptor (TLR) genes in mammals. BMC Evol Bio 11(1):368. doi:10.1186/1471-2148-11-368
Werling D, Jann OC, Offord V, Glass EJ, Coffey TJ (2009) Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol 30(3):124–130. doi:10.1016/j.it.2008.12.001
John T (2011) The MHC, disease and selection. Immunol Lett 137(1–2):1–8. doi:10.1016/j.imlet.2011.01.002
Rebl A, Siegl E, Köllner B, Fischer U, Seyfert H-M (2007) Characterization of twin toll-like receptors from rainbow trout (Oncorhynchus mykiss): evolutionary relationship and induced expression by Aeromonas salmonicida salmonicida. Dev Comp Immunol 31(5):499–510. doi:10.1016/j.dci.2006.08.007
Stafford JL, Ellestad KK, Magor KE, Belosevic M, Magor BG (2003) A toll-like receptor (TLR) gene that is up-regulated in activated goldfish macrophages. Dev Comp Immunol 27(8):685–698. doi:10.1016/s0145-305x(03)00041-7
Xiao X, Qin Q, Chen X (2011) Molecular characterization of a toll-like receptor 22 homologue in large yellow croaker (Pseudosciaena crocea) and promoter activity analysis of its 5′-flanking sequence. Fish Shellfish Immunol 30(1):224–233. doi:10.1016/j.fsi.2010.10.014
Oshiumi H, Tsujita T, Shida K, Matsumoto M, Ikeo K, Seya T (2003) Prediction of the prototype of the human Toll-like receptor gene family from the pufferfish, Fugu rubripes, genome. Immunogenetics 54(11):791–800. doi:10.1007/s00251-002-0519-8
Nishimura M, Naito S (2005) Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull 28(5):886–892. doi:10.1248/bpb.28.886
Baoprasertkul P, Xu P, Peatman E, Kucuktas H, Liu Z (2007) Divergent toll-like receptors in catfish (Ictalurus punctatus): TLR5S, TLR20, TLR21. Fish Shellfish Immunol 23(6):1218–1230. doi:10.1016/j.fsi.2007.06.002
Watzke J, Schirmer K, Scholz S (2007) Bacterial lipopolysaccharides induce genes involved in the innate immune response in embryos of the zebrafish (Danio rerio). Fish Shellfish Immunol 23(4):901–905. doi:10.1016/j.fsi.2007.03.004
Brownlie R, Zhu J, Allan B, Mutwiri GK, Babiuk LA, Potter A, Griebel P (2009) Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol Immunol 46(15):3163–3170. doi:10.1016/j.molimm.2009.06.002
Hirono I, Takami M, Miyata M, Miyazaki T, Han H-J, Takano T, Endo M, Aoki T (2004) Characterization of gene structure and expression of two toll-like receptors from Japanese flounder, Paralichthys olivaceus. Immunogenetics 56(1):38–46. doi:10.1007/s00251-004-0657-2
Matsuo A, Oshiumi H, Tsujita T, Mitani H, Kasai H, Yoshimizu M, Matsumoto M, Seya T (2008) Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J Immunol 181(5):3474–3485
Meijer AH, Gabby Krens SF, Medina Rodriguez IA, He S, Bitter W, Ewa Snaar-Jagalska B, Spaink HP (2004) Expression analysis of the toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol 40(11):773–783. doi:10.1016/j.molimm.2003.10.003
Acknowledgments
Mr. Sundaram’s PhD is funded by a scholarship from the University of Nordland (Norway). We are grateful to Dr. Alessia Giannetto, Dr. Kazue Nagasawa, Ms. Catarina Campos and Mr. Spyros Kollias for their help in laboratory work and fish husbandry.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sundaram, A.Y.M., Consuegra, S., Kiron, V. et al. Positive selection pressure within teleost toll-like receptors tlr21 and tlr22 subfamilies and their response to temperature stress and microbial components in zebrafish. Mol Biol Rep 39, 8965–8975 (2012). https://doi.org/10.1007/s11033-012-1765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1765-y