Abstract
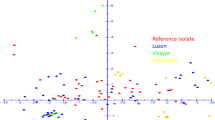
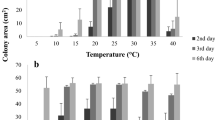
The effects of temperature and pH on the growth of 45 Hungarian Macrophomina phaseolina isolates from different locations and hosts were compared on the basis of their genetic diversity. One Spanish and two Serbian isolates were also included in the experiment. The most favourable temperature regimes for the development of the isolates ranged between 25 and 35°C. The optimal pH for the pathogen varied between 4.0 and 6.0, but growth was observed on potato dextrose agar even at pH values of 3.0, 7.0 and 8.0. RAPD analysis with 13 different primer pairs generated 148 unambiguous bands. RFLP analysis involving 8 different restriction endonucleases was performed on a 1550 bp fragment of the rDNA region containing internal transcribed spacers (ITS1, ITS2), the 5.8S rDNA and part of the 25S rDNA. The greatest genetic distance values were obtained for three isolates, two from Hungary and one from Spain, which had similar values, but were quite distinct from all the others. A strong positive correlation was observed between the genetic distances and the growth parameters measured at various temperatures, and between the geographical data and the growth data sets at different pH values, but the correlation was less strong in the latter case. While Hungarian M. phaseolina populations are thought to reproduce clonally, the present results indicate the coexistence of different haplotypes in this area, and besides the geographical dominance of a given haplotype it was found that a closer genetic relationship might exist between spatially distinct haplotypes.






Similar content being viewed by others
References
Purkayastha S, Kaur B, Dilbaghi N, Chaudhury A (2006) Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCR-based molecular markers. Plant Pathol 55:106–116
Short GE, Wyllie TD, Bristow PR (1980) Survival of Macrophomina phaseolina in soil and in residue of soybean. Phytopathology 70:13–17
Singh RS, Chohan JS (1982) Physio-pathological studies of Macrophomina phaseolina causing charcoal rot in muskmelon. Indian J Mycol Plant Pathol 12:81–82
Das ND (1988) Effect of different sources of carbon nitrogen and temperature on the growth and sclerotial production of Macrophomina phaseolina (Tassi) Goid., causing root rot/charcoal rot disease of castor. Indian J Plant Pathol 6:97–98
Maholay MN (1992) Macrophomina seed and pod rot of butter bean (Phaseolus lunatus L.). Indian J Mycol Plant Pathol 22:220–226
Ratnoo RS, Bhatnagar MK (1991) Effect of temperature and pH on growth and sclerotia formation of Macrophomina phaseolina. Indian J Mycol Plant Pathol 21:279–280
Su G, Suh SO, Schneider RW, Russin JS (2001) Host specialization in the charcoal rot fungus, Macrophomina phaseolina. Phytopathology 91:120–126
Aboshosha SS, Atta Alla SI, El-Korany AE, El-Argawy E (2007) Characterization of Macrophomina phaseolina isolates affecting sunflower growth in El-Behera Governorate. Egypt Int J Agri Biol 9:807–815
Das IK, Fakrudin B, Arora DK (2008) RAPD cluster analysis and chlorate sensitivity of some Indian isolates of Macrophomina phaseolina from sorghum and their relationships with pathogenicity. Microbiol Res 163:215–224
Mayek-Perez N, Lopez-Castaneda C, Gonzales-Chavira M, Garcia-Espinosa R, Acosta-Gallegos J, de la Vega OM, Simpson J (2001) Variability of Mexican isolates of Macrophomina phaseolina based on pathogenesis and AFLP genotype. Phys Mol Plant Path 59:257–264
Reyes-Franco MC, Hernández-Delgado S, Beas-Fernández R, Medina-Fernández M, Simpson J, Mayek-Pérez N (2006) Pathogenic and genetic variability within Macrophomina phaseolina from Mexico and other countries. J Phytopathol 154:447–453
Rajkumar FB, Kuruvinashetti MS (2007) Genetic variability of sorghum charcoal rot pathogen (Macrophomina phaseolina) assessed by random DNA markers. Plant Pathol J 23:45–50
Omar MR, Abd-Elsalam KA, Aly AA, El-Samawaty AMA, Verreet JA (2007) Diversity of Macrophomina phaseolina from cotton in Egypt: analysis of pathogenicity, chlorate phenotypes and molecular characterization. J Plant Dis Protect 114:196–204
Almeida AMR, Abdelnoor RV, Arrabal Arias CA, Carvalho VP, Jacoud Filho DS, Marin SRR, Benato LC, Pinto MC, Carvalho CGP (2003) Genetic diversity among Brazilian isolates of Macrophomina phaseolina revealed by RAPD. Fitopatol Bras 28:279–286
Chase TE, Jiang Y, Mihail J (1994) Molecular variability in Macrophomina phaseolina. Phytopathology 84:1149
Williams JGK, Hanafey MK, Rafalski JA, Tingey SV (1993) Genetic analysis using random amplified polymorphic DNA markers. Method Enzymol 218:704–740
Joshi C, Zhou H, Huang X, Chiang VL (1997) Context sequences of translation initiation codon in plants. Plant Mol Biol 35:993–1001
Sawant SV, Singh PK, Gupta SK, Madnala R, Tuli R (1999) Conserved nucleotide sequences in highly expressed genes in plants. J Genet 78:123–131
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27:86–93
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Dice LR (1945) Measuring of amount of ecological association between species. Ecology 26:297–302
Van de Peer Y, De Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environments. Comput Appl Biosci 10:569–570
Jaccard P (1908) Nouvelles recherchés, sur la distribution florale. Bull Soc Vaud Sci Natur 4:223–270
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Rehner SA, Samuels GJ (1995) Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and status of their anamorphs. Can J Bot 73:S816–S823
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, pp 315–322
Wolinoska J, Spaak P, Koerner H, Petrusek A, Seda J, Giessler S (2011) Transmission mode affects the population genetic structure of Daphnia parasites. J Evol Biol 24:265–273
Seadah AAA, Shikh MEE (2008) RAPD typing of, Aspergillus chevalieri Aspergillus nidulans, Aspergillus tetrazonus (quadrilineatus) and their teleomorphs using 5′-d[AACGCGCAAC]-3′ and 5′-d[CCCGTCAGCA]-3′ primers. Mol Biol Rep 35:89–95
Awan MS, Tabbasam N, Ayub N, Babar ME, Rahman MU, Rana SM, Rajoka MI (2011) Gamma radiation induced mutagenesis in Aspergillus niger to enchance its microbial fermentation activity for industrial enzyme production. Mol Biol Rep 38:1367–1374
Dhingra OD, Sinclair JB (1978) Biology and pathology of Macrophomina phaseolina. Imprensa Universitaria, Universidade Federal de Vicosa
Manici LM, Caputo F, Cerato C (1995) Temperature responses of isolates of Macrophomina phaseolina from different climatic regions of sunflower production in Italy. Plant Dis 79:834–838
Békési P (2007) Sunflower diseases in 2007. Agrofórum 18:17–19
Mihail JD (1989) Macrophomina phaseolina: spatio-temporal dynamics on inoculum and of disease in a highly susceptible crop. Phytopathology 79:848–855
Mihail JD, Taylor SJ (1995) Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production and chlorate utilization. Can J Bot 73:1596–1603
Mayek-Perez N, Garcia-Espinosa R, Lopez-Castaneda C, Acosta-Gellegos JA, Simpson J (2002) Water relations histopathology and growth of common bean (Phaseolus vulgaris L.) during pathogenesis of Macrophomina phaseolina under drought stress. Physiol Mol Plant Pathol 60:185–195
Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE (2006) Climate change effects on plant disease: genomes to ecosystems. Annu Rev Phytopathol 44:489–509
Jimenéz-Díaz RM, Blanco-López MA, Sackston WE (1983) Incidence and distribution of charcoal rot of sunflower caused by Macrophomina phaeolina in Spain. Plant Dis 67:1033–1036
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathology 7:1–16
Saleh AA, Ahmed HU, Todd TC, Travers SE, Zeller KA, Leslie JF, Garrett KA (2010) Relatedness of Macrophomina phaseolina isolates from tallgrass prairie, maize, soybean and sorghum. Mol Ecol 19:79–91
Stevens RM, Douglas T (1994) Distribution of grapevine roots and salt under drip and fullground cover microjet irrigation systems. Irrigat Sci 15:147–152
Olaya G, Abawi GS, Barnard J (1996) Influence of water potential on survival of microsclerotia in soil and on colonization of bean stem segments by Macrophomina phaseolina. Plant Dis 80:1351–1354
Bruton BD, Biles CL, Dunlap JR (1995) Nutrient utilization of Macrophomina phaseolina: a chromogenic isolate from cantaloupe fruit. Subtrop Plant Sci 47:46–52
Nischwitz C, Olsen M, Rasmussen S (2004) Effect of irrigation type on inoculums density of Macrophomina phaseolina in melon fields in Arizona. J Phytopathol 152:133–137
Monga D, Rathore SS, Mayee CD, Sharma TR (2004) Differentation of isolates of cotton root rot pathogens Rhizoctonia solani and R. bataticola using pathogenicity and RAPD markers. J Plant Biochem Biotechnol 13:135–139
Kupriyanova NS, Timofeeva MYa (1988) 32S pre-RNA processing: a dynamic model for interaction with U3RNA and structural rearrangements of spacer regions. Mol Biol Rep 13:91–96
Lindahl L, Zengel JM (1995) RNase MRP and rRNA processing. Mol Biol Rep 22:69–73
Tollervey D (1995) Genetic and biochemical analysis of yeast RNase MRP. Mol Biol Rep 22:75–79
Poczai P, Hyvönen J (2010) Nuclear ribosomal spacer regions in plant phylogenetics: problems and prospects. Mol Biol Rep 37:1897–1912
Coleman AW (2003) ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet 19:370–375
Ciarmello LF, Piccirillo P, Pontecorvo G, De Luca A, Kafantaris I, Woodrow P (2011) A PCR based SNPs marker for specific characterization of English walnut (Juglans regia L.) cultivars. Mol Biol Rep 38:1237–1249
Margam VM, Coates BS, Ba MN, Sun W, Binso-Dabire CL, Baoua I, Ishiyaku MF, Shuke JT, Hellmich RL, Covas FG, Ramasamy S, Armstrong J, Pittendrigh BR, Murdock LL (2011) Geographic distribution of phylogenetically-distinct legume pod borer, Maruca vitrata (Lepidoptera: Pyraloidea: Crambidae). Mol Biol Rep 38:893–903
Deng W, Xi D, Mao H, Wanapat M (2008) The use of molecular techniques based on ribosomal RNA and DNA for rumen microbial ecosystem studies: a review. Mol Biol Rep 35:265–274
Wang H, Sun H, Kwon W-S, Jin H, Yang D-C (2010) A PCR-based SNP marker for specific authentication of Korean ginseng (Panax ginseng) cultivar “Chunpoong”. Mol Biol Rep 37:1053–1057
Pamidimarri DVNS, Chattopadhyay B, Reddy MP (2009) Genetic divergence and phylogenetic analysis of genus Jatropha based on nuclear ribosomal DNA ITS sequence. Mol Biol Rep 36:1929–1935
Anderson JB, Kohn LM (1995) Clonality in soilborne, plantpathogenic fungi. Annu Rev Phytopathol 33:369–391
Crous PW, Slippers B, Wingfield M, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Almeida ÁMR, Sosa-Gomez DR, Binneck E, Marin SRR, Zucchi MI, Abdelnoor RV, Souto ER (2008) Effect of crop rotation on specialization and genetic diversity of Macrophomina phaseolina. Trop Plant Pathol 33:257–264
Baird RE, Wadl PA, Allen T, McNeill D, Wang X, Moulton JK, Rienhart TA, Abbas HK, Shier T, Trigiano RN (2010) Variability of United States isolates of Macrophomina phaseolina based on simple sequence repeats and cross genus transferability to related genera within Botryosphaeriaceae. Mycopathologia 170:169–180
Carlile MJ (1986) Genetic exchange and gene flow: their promotion and prevention. In: Rayner ADM, Moore D (eds) Evolutionary biology of the fungi. Cambridge University Press, UK, Cambridge, pp 203–214
Rossman AY, Palm-Hernández ME (2008) Systematics of plant pathogenic fungi: why it matters. Plant Dis 92:1376–1386
Jones RW, Canada S, Wang H (1998) Highly variable minichromosomes and highly conserved endonuclease genes in the phytopathogenic fungus Macrophomina phaseolina. Can J Bot 76:694–698
Acknowledgments
Thanks are due to all those who assisted in collecting the samples and to Dr. Leire Molinero-Ruiz, Dr. Vera Stojsi and Dr. Ferenc Bagi for the Spanish and Serbian isolates. Péter Poczai gratefully acknowledges support from the Hungarian State Eötvös Research Grant (MÖB/90-1/2011). The second author gratefully acknowledges support from the Hungarian Academy of Sciences through the “Bolyai János” research fellowship. Thanks are due to Barbara Harasztos for revising the manuscript linguistically.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Csöndes, I., Cseh, A., Taller, J. et al. Genetic diversity and effect of temperature and pH on the growth of Macrophomina phaseolina isolates from sunflower fields in Hungary. Mol Biol Rep 39, 3259–3269 (2012). https://doi.org/10.1007/s11033-011-1094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1094-6