Abstract
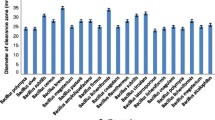
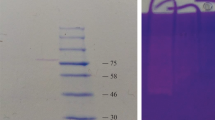
Proteases have prospective financial and environment-friendly applications; hence attention is focused currently on the finding of new protease producing microorganism so as to meet the requirements of industry. A thermophilic bacterial strain producing extracellular protease activity was isolated from soil and identified as Bacillus cereus by analysis of 16S rRNA. Protease production by the microorganism was improved by studying the impact of the type of nitrogen and carbon source, fermentation period, growth temperature and initial pH of the culture medium in cultivation optimization experiments. The enzyme was purified to homogeneity in two step procedure involving Sephadex G-75 and Q-Sepharose chromatography. The molecular weight of purified enzyme was found to be 58 kDa by SDS-PAGE. Protease exhibited a pH and temperature optima of 7.5 and 60°, respectively. The enzyme was active in the pH range of 6.0–9.0 and stable up to 70°C. Histological analysis of protease treated goat and cow skin pelts showed complete removal of non leather forming structures such as hair shaft, hair follicles and glandular structures. The protease showed the stain removing property from blood stained cotton cloth and found to be compatible with six commercially available detergents. The protease could release peptides from natural proteins after digestion of coagulated egg albumin and blood clot.







Similar content being viewed by others
References
Sookkheo B, Sinchaikul S, Phutrakul S, Chen ST (2000) Purification and characterization of the highly thermostable proteases from Bacillus stearothermophilus TLS33. Protein Exp Purif 20:142–151
Beg QK, Sahai V, Gupta R (2003) Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem 39:203–209
Rao MB, Tanksale AM, Mohini SG, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–602
Kumar R, Balaji S, Uma TS, Mandal AB, Sehgal PK (2010) Optimization of influential parameters for extracellular keratinase production by Bacillus subtilis (MTCC9102) in solid state fermentation using horn meal—a biowaste management. Appl Biochem Biotechnol 160:30–39
Cheng-gang CAI, Ji-shuang C, Jiong-jiong QI, Yun Y, Xiao-dong Z (2008) Purification and characterization of keratinase from a new Bacillus subtilis strain. J Zhejiang Univ Sci 9:713–720
Zhu HY, Tian Y, Hou YH, Wang T (2009) Purification and characterization of the cold-active alkaline protease from marine cold-adaptive Penicillium chrysogenum. Mol Biol Rep 36:2169–2174
Cowan DA (1994) Industrial Enzymes. In: Moses V, Cape RE (eds) Biotechnology—the science and the business. Harwood Academic Publishers, Switzerland, pp 326–328
Mizuho N, Tomiko A, Etsuko K, Mam M, Wakako E, Tomoko T, Akio O, Keiko A, Junko F (2008) Application of an Aspergillus saitoi protease preparation to soybean curd to modify its functional and rheological properties. Biosci Biotechnol Biochem 72:587–590
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–17
Han HW, Srinivasan VR (1968) Isolation and characterization of cellulose utilizing bacterium. Appl Microbiol 16:1140–1145
Kunitz M (1947) Crystalline soybean trypsin inhibitor. J Gen Physiol 30:291–310
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Shafee N, Sayangku NA, Raja NZ B, Mahiran AR, Abu Bakar S (2005) Optimization of environmental and nutritional conditions for the production of alkaline protease by a newly isolated bacterium Bacillus cereus strain 146. J Appl Sci Res 1:21–28
Chu IM, Lee CLi TS (1992) Production and degradation of alkaline protease in batch cultures of Bacillus subtilis ATCC 14416. Enz Microbial Technol 14:755–761
Nilegaonkar SS, Zambare V, Kanekar PP, Dhakephalkar PK, Sarnai SS (2007) Production and partial characterization of dehairing protease from Bacillus cereus MCM B-326. Biores Technol 98:1238–1245
El-Safey EM, Abdul-Raouf (2004) Production, purification and characterization of protease enzyme from Bacillus subtilis. International Conferences for Development and the Environment in the Arab World, Assiut University
Chellappan S, Jasmin C, Soorej M, Basheer KK, Elyas SGB, Chandrasekaran M (2006) Production, purification and partial characterization of a novel protease from marine Engyodontium album BTMFS10 under solid state fermentation. Process Biochem 41:956–961
da Silva CRD, Andria BD, Meire LL (2007) Effect of the culture conditions on the production of an extracellular protease by thermophilic Bacillus sp. and some properties of the enzymatic activity. Braz J Microbiol 38:171–173
Guangrong H, Dai D, Hu W, Jiang J (2008) Optimization of medium composition for thermostable protease production by Bacillus sp. HS08 with a statistical method. Afr J Biotechnol 7:1115–1122
Morita Y, Kenji K, Quamrul H, Toshifumi S, Yuji M, Kenji Y, Eiichi T (1997) Purification and characterization of a cold-active protease from psychrotrophic Serratia marcescens AP3801. J Am Oil Chemists Soc 74:1377–1383
Doddapaneni KK, Tatineni R, Vellanki RN, Rachcha S, Anabrolu N, Narakuti V, Mangamoori LK (2009) Purification and characterization of a solvent and detergent-stable Bacillus cereus. Microbiol Res 164:383–390
Ghorbel-Frikha B, Sellami-Kamoun A, Fakhfakh N, Haddar A, Manni L, Nasri M (2005) Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J Ind Microbiol Biotechnol 32:186–194
Chaplin MF, Bucke C (1990) Enzyme technology. Cambridge University Press, Cambridge
Rao K, Lakshmi N (2007) Alkaline protease from Bacillus firmus 7728. Afr J Biotechnol 6:2493–2496
Reddy L, Wee YJ, Ryu HW (2008) Purification and characterization of anorganic solvent and detergent-tolerant novel protease produced by Bacillus sp. RKY3. J Chemical Technol Biotechnol 83:1526–1533
Joo HS, Kumar CG, Park GC, Paik SR, Chang CS (2003) Oxidant and SDS-stable alkaline protease from Bacillus clausii I-52: production and some properties. J Appl Microbiol 95:267–271
Pawar R, Zambare V, Barve S, Paratkar G (2009) Application of protease isolated from Bacillus sp. 158 in enzymatic cleansing of contact lenses. Biotechnology 8:276–280
Kaur S, Vohra RM, Kapoor M, Beg QK, Hoondal GS (2001) Enhanced production and characterization of a highly thermostable alkaline protease from Bacillus sp. P-2. World J Microbiol Biotechnol 17:125–129
Kamoun AL, Haddar A, Ali NE, Frikha BG, Kanoun S, Nasri M (2008) Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbial Res 163:299–306
Thanikaivelan P, Bharath CK, Saravanabhavan S, Chandrasekaran B, Rao JR, Chandrababu NK, Nair BU (2007) Integrated hair removal and fiber opening process using mixed enzymes. Clean Technol Environ Policy 9:61–68
Mukhtar H, Haq I (2008) Production of alkaline protease by Bacillus subtilis and its application as a depilating agent in leather processing. Pak J Bot 40:673–1679
Pillai P, Archana G (2008) Hide depilation and feather disintegration studied with keratinolytic serine protease from a novel Bacillus subtilis isolate. Appl Microbiol Biotechnol 78:643–650
Rao CS, Sathish T, Ravichandra P, Prakasham RS (2009) Characterization of thermo- and detergent stable serine protease from isolated Bacillus circulans and evaluation of eco-friendly applications. Process Biochem 44:262–268
Sivasubramanian S, Murali BM, Rajaram A, Puvanakrishnan R (2008) Ecofriendly lime and sulphide free enzymatic dehairing of skins and hides using a bacterial alkaline protease. Chemophere 70:1015–1024
Macedo AJ, Da Silva W, Gava R, Driemeier D, Henriques JAP, Termignoni C (2005) Novel keratinase from Bacillus subtillus S14 exhibiting remarable dehairing capabilities. Appl Environ Microbiol 71:594–596
Thanikaivelan P, Rao JR, Nair BU, Ramasami T (2004) Progress and recent trends in biotechnological methods for leather processing. Trends Biotechnol 22:181–188
Zambare VP, Nilegaonkar SS, Kanekar PP (2007) Production of an alkaline protease by Bacillus cereus MCM B-326 and its application as a dehairing agent. World J Microbiol Biotechnol 23:1569–1574
Najafi MF, Dileep D, Deepti D (2005) Potential application of protease isolated from Pseudomonas aeruginosa PD100. Electron J Biotechnol 8:197–203
Banik RM, Prakash M (2004) Laundry detergent compatibility of the alkaline protease from Bacillus cereus. Res Microbiol 159:135–140
Brown DA, Cameron BA, Meyer SS (1993) A survey of commercial laundry detergent- How effective they are? Part 1: Powders. J Consumer Studies Home Econom 171:145–152
Shikha AS, Darmwal NS (2007) Improved production of alkaline proteases from a mutant form of alkalophilic Bacillus pantotheneticus using molasses as a substrate. Biores Technol 98:881–885
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saleem, M., Rehman, A., Yasmin, R. et al. Biochemical analysis and investigation on the prospective applications of alkaline protease from a Bacillus cereus strain. Mol Biol Rep 39, 6399–6408 (2012). https://doi.org/10.1007/s11033-011-1033-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1033-6