Abstract
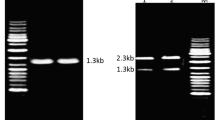
The production and purification of a calcium-dependent protease by Bacillus cereus BG1 were studied. The production of the protease was found to depend specifically on the calcium concentration in the culture medium. This suggests that this metal ion is essential for the induction of protease production and/or stabilisation of the enzyme after synthesis. The calcium requirement is highly specific since other metal ions (such as Mg2+ and Ba2+, which both activate the enzyme) are not able to induce protease production. The most appropriate medium for growth and protease production comprises (g L−1) starch 5, CaCl2 2, yeast extract 2, K2HPO4 0.2 and KH2PO4 0.2. The protease of BG1 strain was purified to homogeneity by ultrafiltration, heat treatment, gel filtration on Sephacryl S-200, ion exchange chromatography on DEAE-cellulose and, finally, a second gel filtration on Sephacryl S-200, with a 39-fold increase in specific activity and 23% recovery. The molecular weight was estimated to be 34 kDa on SDS-PAGE. The optimum temperature and pH of the purified enzyme were determined to be 60°C and 8.0, respectively, in 100 mM Tris-HCl buffer + 2 mM CaCl2.









Similar content being viewed by others
References
Bradford M (1976) A rapid and sensitive method for the quantification of microorganism quantities of protein utilizing the principle of dye binding. Anal Biochem 72:248–254
Do Nascimento WCA, Martins MLL (2004) Production and properties of an extracellular protease from thermophilic Bacillus sp. Braz J Microbiol 35:91–96
Donovan WP, Tan Y, Slaney AC (1997) Cloning of the nprA gene for neutral protease A of Bacillus thuringiensis and effect of in vivo deletion of nprA on insecticidal crystal protein. Appl Environ Microbiol 63:2311–2317
Drucker H (1972) Regulation of exocellular protease in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol 110:1041–1049
Ferrero MA, Castro GR, Abate CM, Baigori MD, Sineriz F (1996) Thermostable alkaline proteases of Bacillus licheniformis MIR 29: isolation, production and characterization. Appl Microbiol Biotechnol 45:327–332
Frankena J, Koningstein GM, van Verseveld HW, Stouthamer AH (1986) Effect of different limitations in chemostat cultures on growth and production of exocellular protease by Bacillus licheniformis. Appl Microbiol Biotechnol 24:106–112
Fukushima Y, Itoh H, Fukase T, Motai H (1989) Continuous protease production in a carbon-limited chemostat culture by salt tolerant Aspergillus oryzae. Appl Microbiol Biotechnol 30:604–608
Gessesse A, Gashe BA (1997) Production of alkaline protease by an alkaliphilic bacteria isolated from an alkaline soda lake. Biotechnol Lett 19:479–481
Ghorbel B, Sellami-Kamoun A, Nasri M (2003) Stability studies of protease from Bacillus cereus BG1. Enzyme Microb Technol 32:513–518
Giesecke UE, Bierbaum G, Rudde H, Spohn U, Wandrey C (1991) Production of alkaline protease with Bacillus licheniformis in a controlled fed-batch process. Appl Microbiol Biotechnol 35:720–724
Godfrey TA, Reichelt P (1985) Industrial enzymology: the application of enzymes in industry. The Nature Press, London
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:13–32
Hayano K, Takeuchi M, Ichishima E (1987) Characterization of a metalloproteinase component extracted from soil. Biol Fertil Soils 4:179–183
Johnvesly B, Naik GR (2001) Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem 37:139–144
Kelly CT, Fogarty WM (1976) Microbial alkaline enzymes. Process Biochem 11:3–9
Kembhavi AA, Kulkarni A, Pant AA (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No.64. Appl Biochem Biotechnol 38:83–92
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liao CHS, McCallus DE (1998) Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl Environ Microbiol 64:914–921
Mabrouk SS, Hashem AM, El-Shayeb NMA, Ismail AMS, Abdel-Fattah AF (1999) Optimisation of alkaline protease productivity by Bacillus licheniformis ATCC 21415. Bioresour Technol 69:155–159
Mehrotra S, Pandey PK, Gaur R, Darmwal NS (1999) The production of alkaline protease by a Bacillus species isolate. Bioresour Technol 67:201–203
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 431–435
Priest FG (1977) Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev 41:711–753
Secades P, Alvarez B, Guijarro JA (2001) Purification and characterization of a Psychrophilic, calcium-induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Appl Environ Microbiol 67:2436–2444
Singh J, Batra N, Sobti RC (2001) Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Process Biochem 36:781–785
Stark W, Pauptit RA, Wilson KS, Jansonius JN (1992) The structure of neutral protease of Bacillus cereus at 0.2 nm resolution. Eur J Biochem 207:781–791
Zukowski MM (1992) Production of commercially valuable products. In: Doi RH, McGloughlin M (eds) Biology of bacilli: application to industry. Butterworth-Heinemann, London, pp 311–337
Acknowledgements
We are grateful to Mr. A. Hajji from the Engineering School of Sfax for his help with English. This work was funded by the “Ministère de la Recherche Scientifique, de la Technologie et du Développement des Compétences, Tunisie”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbel-Frikha, B., Sellami-Kamoun, A., Fakhfakh, N. et al. Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J IND MICROBIOL BIOTECHNOL 32, 186–194 (2005). https://doi.org/10.1007/s10295-005-0228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0228-z