Abstract
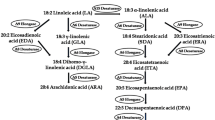
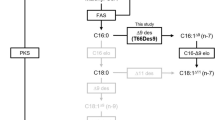
Fatty acid composition of fungi is analysed through the gas chromatography technique. With specific activity a novel enzyme △6-fatty acid desaturase was screened and isolated from Rhizopus nigricans. In this study R. nigricans was identified as a fungal species that produced plentiful γ-linolenic acid. A 1,475 bp full-length cDNA, designated as RnD6D here, with high homology to fungal △6-fatty acid desaturase genes was isolated from R. nigricans using reverse transcription polymerase chain reaction and rapid amplification of cDNA ends methods. Sequence analysis indicated that this cDNA sequence had an open reading frame of 1,380 bp encoding a deduced polypeptide of 459 amino acids. Bioinformatics analysis characterized the putative RnD6D protein as a typical membrane-bound desaturase, including three conserved histidine-rich motifs, hydropathy profile and a cytochrome b5-like domain in the N-terminus. The corresponding genomic sequence of RnD6D was 1,689 bp with 4 introns, which was at least one intron more than other fungal △6-fatty acid desaturase genes. To elucidate the function of this novel putative desaturase, the coding sequence was expressed in Saccharomyces cerevisiae strain INVScl. A novel peak corresponding to γ-linolenic acid methyl ester standards was detected with the same retention time, which was absent in the cell transformed with empty vector. The result demonstrated that the coding produced △6-fatty acid desaturase activity of RnD6D which led to the accumulation of γ-linolenic acid. The functionally active RnD6D gene cloned here defined a novel △6-fatty acid desaturase from R. nigricans.





Similar content being viewed by others
References
Kapoor R, Huang YS (2006) Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol 7:531–535. doi:10.2174/138920106779116874
de Gyves EM, Sparks CA, Sayanova O et al (2004) Genetic manipulation of gamma-linolenic acid (GLA) synthesis in a commercial variety of evening primrose (Oenothera sp.). Plant Biotechnol J 2(4):351–358
Philips JC, Huang YS (1996) Natural sources and biosynthesis of γ-linolenic acid: an overview. In: Huang YS, Milles DE (eds) γ-Linolenic acid: metabolism and its roles in nutrition and medicine. AOCS Press, Champaign
Huang YS, Chaudhary S, Thurmond JM et al (1999) Cloning of delta 12- and delta 6-desaturases from Mortierella alpina and recombinant production of gamma-linolenic acid in Saccharomyces cerevisiae. Lipids 34:649–659. doi:10.1007/s11745-999-0410-8
Morrison MR, Smith M (1964) Preparation of fatty acid methyl esters and dimethyl acetal from lipids with boron trifluride-/methanol. J Lipid Res 5:600–608
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi:10.1016/0003-2697(87)90021-2
Elble R (1992) A simple and efficient procedure for transformation of yeasts. Biotechniques 13:18–20
Napier JA, Hey SJ, Lacey DJ et al (1998) Identification of a Caenorhabditis elegans ∆6-fatty-acid-desaturase by heterologous expression in Saccharomyces cerevisiae. Biochem J 330:611–614
Domergue F, Lerchl J, Zahringer U et al (2002) Cloning and functional characterization of Phaeodactylum tricornutum front-end desaturases involved in eicosapentaenoic acid biosynthesis. Eur J Biochem 269:4105–4113. doi:10.1046/j.1432-1033.2002.03104.x
Sayanova O, Smith MA, Lapinskas P et al (1997) Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of D 6-desaturated fatty acids in transgenic tobacco. Proc Nat Acad Sci USA 94, 4211–4216
Aki T, Shimada Y, Inagaki K et al (1999) Molecular cloning and functional characterization of rat delta-6 fatty acid desaturase. Biochem Biophys Res Commun 255:575–579. doi:10.1006/bbrc.1999.0235
Laoteng K, Mannontarat R, Tanticharoen M et al (2000) Delta(6)-desaturase of Mucor rouxii with high similarity to plant delta(6)-desaturase and its heterologous expression in Saccharomyces cerevisiae. Biochem Biophys Res Commun 279:17–22. doi:10.1006/bbrc.2000.3856
Napier JA, Sayanova O, Sperling P et al (1999) A growing family of cytochrome b5-domain fusion proteins. Trends Plant Sci 4:2–4. doi:10.1016/S1360-1385(98)01357-0
Shanklin J, Whittle E, Fox BG (1994) Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:127871–127894. doi:10.1021/bi00209a009
Sperling P, Zähringer U, Heinz E (1998) A sphingolipid desaturase from higher plants identification of a new cytochrome b5 fusion protein: Identification of a new cytochrome b5 fusion protein. J Biol Chem 273:28590–28596. doi:10.1074/jbc.273.44.28590
Sayanova O, Beaudoin F, Libisch B et al (2001) Mutagenesis and heterologous expression in yeast of a plant Delta6-fatty acid desaturase. J Exp Bot 52:1581–1585. doi:10.1093/jexbot/52.360.1581
Macartey A, Maresca B, Cossins AR (1994) Temperature adaptation of biological membranes (Cossins AR ed.). Portland Press, London, pp 63–76
Hong H, Datla N, Reed DW et al (2002) High-level production of gamma-linolenic acid in Brassica juncea using a Delta6 desaturase from Pythium irregulare. Plant Physiol 129:354–362. doi:10.1104/pp.001495
Acknowledgments
This research was supported by the Key Project of Chinese Ministry of Education, the S&T Research Program of Chong Qing Municipal Education Commission, China national “948” Program and China national “863” Program etc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, H., Li, JN., Chai, YR. et al. Identification and characterization of a novel ∆6-fatty acid desaturase gene from Rhizopus nigricans . Mol Biol Rep 36, 2291–2297 (2009). https://doi.org/10.1007/s11033-009-9447-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9447-0