Abstract
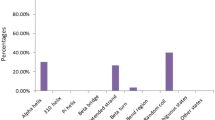
A gene encoding a putative asparagine synthetase (AS; EC 6.3.5.4) has been isolated from common bean (Phaseolus vulgaris). A 2.4 kb cDNA clone of this gene (PVAS3) encodes a protein of 570 amino acids with a predicted molecular mass of 64,678 Da, an isoelectric point of 6.45, and a net charge of −5.9 at pH 7.0. The PVAS3 protein sequence conserves all the amino acid residues that are essential for glutamine-dependent AS, and PVAS3 complemented an E. coli asparagine auxotroph, that demonstrates that it encodes a glutamine-dependent AS. PVAS3 displayed significant similarity to other AS. It showed the highest similarity to soybean SAS3 (92.9% identity), rice AS (73.7% identity), Arabidopsis ASN2 (73.2%) and sunflower HAS2 (72.9%). A phylogenetic analysis revealed that PVAS3 belongs to class-II asparagine synthetases. Expression analysis by real-time RT-PCR revealed that PVAS3 is expressed ubiquitously and is not repressed by light.






Similar content being viewed by others
References
Waters JK, Hughes BL II, Purcel LC et al (1998) Alanine, not ammonia, is excreted from N2-fixing soybean nodule bacteroids. Proc Natl Acad Sci USA 95:12038–12042. doi:10.1073/pnas.95.20.12038
Pate JS, Atkins CA, White ST et al (1980) Nitrogen nutrition and xylem transport of nitrogen in ureide-producing grain legumes. Plant Physiol 65:961–965
Schubert KR (1986) Products of biological nitrogen fixation in higher plants: synthesis, transport, and metabolism. Annu Rev Plant Physiol 37:539–574. doi:10.1146/annurev.pp.37.060186.002543
Peoples MB, Sudin MN, Herridge DF (1987) The effect of nitrogen source on transport and metabolism of nitrogen in fruiting plants of cowpea (Vigna ungiculata (L.) Walp.). J Exp Bot 38:567–579. doi:10.1093/jxb/38.4.567
Patterson TG, LaRue TA (1983) N2 fixation (C2H2) and ureide content of soybeans: environmental effects and sources sink manipulations. Crop Sci 23:819–824
Schubert KR, Boland MJ (1990) The ureides. In: Miflin BJ, Lea PJ (eds) The biochemistry of plants, vol 16. Academic Press, San Diego, pp 197–283
Hubert R, Simoni RD (1980) Genetic and biochemical studies demonstrating a second gene coding for asparagine synthetase in Escherichia coli. J Bacteriol 142:212–220
Cedar H, Schwartz JH (1969) The asparagine synthetase of Escherichia coli I. Biosynthetic role of the enzyme, purification, and characterization of the reaction products. J Biol Chem 244:4112–4121
Lea PJ, Fowden L (1975) The purification and properties of glutamine-dependent asparagine synthetase isolated from Lupinus albus. Proc R Soc Lond B Biol Sci 192:13–26
Joy KW, Ireland RJ, Lea PJ (1983) Asparagine biosynthesis in pea leaves and the occurrence of an asparagine synthetase inhibitor. Plant Physiol 73:165–168
Huber TA, Streeter JG (1985) Purification and properties of asparagine synthetase from soybean root nodules. Plant Sci 42:9–17. doi:10.1016/0168-9452(85)90022-6
Ta TC, MacDowall FDH, Faris MA (1989) Asparagine synthetase from root nodules of alfalfa. Biochem Cell Biol 67:455–459
Tsai FY, Coruzzi GM (1990) Dark-induced and organ-specific expression of two asparagine synthetase genes in Pisum sativum. EMBO J 9:323–332
Tsai FY, Coruzzi GM (1991) Light represses transcription of asparagine synthetase genes in photosynthetic and non-photosynthetic organs of plants. Mol Cell Biol 11:4966–4972
Davies KM, King GA (1993) Isolation and characterization of a cDNA clone for a harvest-induced asparagine synthetase from Asparagus officinalis. Plant Physiol 102:1337–1340. doi:10.1104/pp.102.4.1337
Lam HM, Peng SS, Coruzzi GM (1994) Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol 106:1347–1357. doi:10.1104/pp.106.4.1347
Chevalier C, Bourgeois E, Just D et al (1996) Metabolic regulation of asparagine synthetase gene expression in maize root tips. Plant J 9:1–11. doi:10.1046/j.1365-313X.1996.09010001.x
Hughes CA, Beard HS, Matthews BF (1997) Molecular cloning and expression of two cDNAs encoding asparagine synthetase in soybean. Plant Mol Biol 33:301–311. doi:10.1023/A:1005784202450
Shi L, Twary SN, Yoshioka H et al (1997) Nitrogen assimilation in alfalfa: isolation and characterization of an asparagine synthetase gene showing enhanced expression in root nodules and dark-adapted leaves. Plant Cell 9:1339–1356
Scofield MA, Lewis WS, Schuster SM (1990) Nucleotide sequence of Escherichia coli asnB and deduced amino acid sequence of asparagine synthetase B. J Biol Chem 265:12895–12902
Osuna D, Gálvez-Valdivieso G, Piedras P et al (2001) Cloning, characterization and mRNA expression analysis of PVAS1, a type I asparagine synthetase gene from Phaseolus vulgaris. Planta 213:402–410. doi:10.1007/s004250000513
Herrera-Rodríguez MB, Carrasco-Ballesteros S, Maldonado JM et al (2002) Three genes showing distinct regulatory patterns encode the asparagine synthetase of sunflower (Helianthus annuus). New Phytol 155:33–45. doi:10.1046/j.1469-8137.2002.00437.x
Osuna D, Gálvez G, Pineda M et al (1999) RT-PCR cloning, characterization and mRNA expression analysis of a cDNA encoding a type II asparagine synthetase in common bean. Biochim Biophys Acta 1445:75–85
Hannahan D (1983) Studies on transformation of E. coli with plasmids. J Mol Biol 166:557–580. doi:10.1016/S0022-2836(83)80284-8
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbour Laboratory, Cold Spring Harbor
Connell C, Fung S, Heiner C et al (1985) Automated DNA sequence analysis. Biotechniques 5:342–348
Joshi CP (1987) Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res 15:9624–9640
Zalkin H, Smith JL (1998) Enzymes utilizing glutamine as an amide donor. Adv Enzymol Relat Areas Mol Biol 72:87–144. doi:10.1002/9780470123188.ch4
Boehlein SK, Walworth ES, Richards NGJ et al (1997) Mutagenesis and chemical rescue indicate residues involved in beta-aspartyl-AMP formation by Escherichia coli asparagine synthetase B. J Biol Chem 272:12384–12392. doi:10.1074/jbc.272.19.12384
Boehlein SK, Walworth ES, Schuster SM (1997) Identification of cysteine-523 in the aspartate binding site of Escherichia coli asparagine synthetase B. Biochemistry 36:10168–10177. doi:10.1021/bi970494l
Richards NGJ, Schuster SM (1998) Mechanistic issues in asparagine synthetase catalysis. Adv Enzymol Relat Areas Mol Biol 72:145–198. doi:10.1002/9780470123188.ch5
Mäntsäla P, Zalkin H (1992) Cloning and sequence of Bacillus subtilis purA and guaA, involved in the conversion of IMP to AMP and GMP. J Bacteriol 174:1883–1990
Reese MG, Harris NL, Eeckman FH (1996) Large scale sequencing specific neural networks for promoter and splice site recognition. In: Hunter L, Klein TE (eds) Biocomputing: proceedings of the 1996 Pacific symposium. World Scientific Publishing Co, Singapore, pp 737–738
Higo K, Ugawa Y, Iwamoto M et al (1999) Plant cis-acting regulatory DNA elements (PLACE) database:1999. Nucleic Acids Res 27:297–300. doi:10.1093/nar/27.1.297
Terzaghi WB, Cashmore AR (1995) Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol 46:445–474. doi:10.1146/annurev.pp.46.060195.002305
Reyes JC, Muro-Pastor MI, Florencio FJ (2004) The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol 134:1718–1732. doi:10.1104/pp.103.037788
Chan CS, Guo L, Shih MC (2001) Promoter analysis of the nuclear gene encoding the chloroplast glyceraldehyde-3-phosphate dehydrogenase B subunit of Arabidopsis thaliana. Plant Mol Biol 46:131–141. doi:10.1023/A:1010602031070
Yanagisawa S (2000) Dof1 and Dof2 transcription factors are associated with expression of multiple genes involved in carbon metabolism in maize. Plant J 21:281–288. doi:10.1046/j.1365-313x.2000.00685.x
Morita A, Umemura T, Kuroyanagi M et al (1998) Functional dissection of a sugar-repressed alpha-amylase gene (Ramy1A) promoter in rice embryos. FEBS Lett 423:81–85. doi:10.1016/S0014-5793(98)00067-2
Robatzeck S, Somssich IE (2002) Targets of AtWRKY6 regulation senescence and pathogen defense. Genes Dev 16:1139–1149. doi:10.1101/gad.222702
Rieping M, Schoffl F (1992) Synergistic effect of upstream sequences, CCAAT box elements, and HSE sequences for enhanced expression of chimaeric heat shock genes in transgenic tobacco. Mol Gen Genet 231:226–232
Lescot M, Déhais P, Thijs G et al (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327. doi:10.1093/nar/30.1.325
Peterman TK, Goodman HM (1991) The glutamine synthetase gene family of Arabidopsis thaliana: light-regulation and differential expression in leaves, roots and seed. Mol Gen Genet 230:145–154. doi:10.1007/BF00290662
Li M, Villemur R, Hussey PJ et al (1993) Differential expression of six glutamine synthetase genes in Zea mays. Plant Mol Biol 23:401–407. doi:10.1007/BF00029015
Waterhouse RN, Smyth AJ, Massonneau A et al (1996) Molecular cloning and characterization of asparagine synthetase from Lotus japonicus: dynamics of asparagine synthesis in N-sufficient conditions. Plant Mol Biol 30:883–897. doi:10.1007/BF00020801
Lam HM, Hsieh MH, Coruzzi GM (1998) Reciprocal regulation of distinct asparagine synthetase genes by light and metabolites in Arabidopsis thaliana. Plant J 16:345–353. doi:10.1046/j.1365-313x.1998.00302.x
Nakamura M, Yamada M, Hirota Y et al (1981) Nucleotide sequence of the asnA gene coding for asparagine synthetase in E. coli K-12. Nucleic Acids Res 9:4669–4676. doi:10.1093/nar/9.18.4669
Ramos F, Wiame JM (1980) Two asparagine synthetases in Sacharomyces cerevisiae. Eur J Biochem 108:373–378. doi:10.1111/j.1432-1033.1980.tb04732.x
Streeter JC (1977) Asparaginase and asparagine transaminase in soybean leaves and root nodules. Plant Physiol 60:235–239
Pfall MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2003–2007. doi:10.1093/nar/29.10.2003
Herrera-Rodriguez MB, Maldonado JM, Perez-Vicente R (2004) Light and metabolic regulation of HAS1, HAS1.1 and HAS2, three asparagine synthetase genes in Helianthus annuus. Plant Physiol Biochem 42:511–518. doi:10.1016/j.plaphy.2004.05.001
Sieciechowicz KA, Joy KW, Ireland RJ (1988) The metabolism of asparagine in plants. Phytochemistry 27:663–671. doi:10.1016/0031-9422(88)84071-8
Wong HK, Chan HK, Coruzzi GM et al (2004) Correlation of ASN2 gene expression with ammonium metabolism in Arabidopsis. Plant Physiol 134:332–338. doi:10.1104/pp.103.033126
Simier P, Delavault P, Demarsy E et al (2005) Characterization of an unusually regulated gene encoding asparagine synthetase in the parasitic plant Striga hermonthica (Scrophulariaceae). Physiol Plant 123:9–20. doi:10.1111/j.1399-3054.2004.00438.x
Lea PJ, Sodek L, Parry MAJ et al (2007) Asparagine in plants. Ann Appl Biol 150:1–26. doi:10.1111/j.1744-7348.2006.00104.x
Herrera-Rodriguez MB, Perez-Vicente R, Maldonado JM (2007) Expression of asparagine synthetase genes in sunflower (Helianthus annuus) under various environmental stresses. Plant Physiol Biochem 45:33–38. doi:10.1016/j.plaphy.2006.12.002
Gálvez-Valdivieso G, Osuna D, Maldonado JM et al (2005) Purification of a functional asparagine synthetase (PVAS2) from common bean (Phaseolus vulgaris), a protein predominatly found in root tissues. Plant Sci 168:89–94. doi:10.1016/j.plantsci.2004.07.014
Acknowledgments
This work was funded by Dirección General de Enseñanza Superior (Grant BIO2006-09366) and by Plan Andaluz de Investigación (CV-0115). E.P.-P. thanks Félix Parra, Santi Peralbo for their teaching and continuous support, Minerva P–P for her invaluable technician help, and Joaquim Culí for his support and suggestions during the last stages of his work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parra-Peralbo, E., Pineda, M. & Aguilar, M. PVAS3, a class-II ubiquitous asparagine synthetase from the common bean (Phaseolus vulgaris). Mol Biol Rep 36, 2249–2258 (2009). https://doi.org/10.1007/s11033-008-9441-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9441-y