Abstract
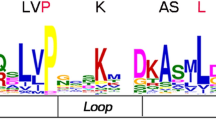
Environmental stresses significantly influence the growth and development of plants. To overcome these stresses, higher plants have evolved a variety of complicated molecular mechanisms. Basic helix−loop−helix (bHLH) transcription factors (TFs) play important roles in plant growth and development in response to environmental stresses. Here, a total of 146 DcbHLH TFs were identified from carrot, based on a genomic and transcriptomic database. Based on the previous classification system of Arabidopsis thaliana, the DcbHLH TFs were divided into 17 subfamilies. Multiple sequence alignment of bHLH conserved domains indicated that 109 DcbHLH proteins were bound to DNA (83 proteins were E-box binders and 52 DcbHLHs were G-box binders). From evolutionary analysis, bHLH TFs selected from plants, metazoans, and fungi demonstrated that the number of bHLH TFs increased during the evolution of these species. The expression profiles of eight DcbHLH genes from subfamily 15 showed differences in three tissues and four abiotic stresses in two carrot cultivars, Junchuanhong and Kurodagosun. This study presented useful information on the structure and function of DcbHLH factors in the regulatory mechanisms of carrot.





Similar content being viewed by others
Abbreviations
- bHLH:
-
Basic helix−loop−helix
- CarrotDB:
-
A genomic and transcriptomic database for carrot
- TF:
-
Transcription factor
- qPCR:
-
Quantitative real-time PCR
- PEG:
-
Polyethylene glycol
References
Alessandro MS, Galmarini CR, Iorizzo M, Simon PW (2013) Molecular mapping of vernalization requirement and fertility restoration genes in carrot. Theor Appl Genet 126:415–423
Atchley WR, Fitch WM (1997) A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci USA 94:5172–5176
Atchley WR, Terhalle W, Dress A (1999) Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J Mol Evol 48:501–516
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37(Web Server issue):W202-8. doi: 10.1093/nar/gkp335
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1158–1203
Budahn H, Barański R, Grzebelus D, Kiełkowska A, Straka P, Metge K, Linke B, Nothnagel T (2014) Mapping genes governing flower architecture and pollen development in a double mutant population of carrot. Front Plant Sci 5:504
Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martinez-Garcia JF, Bilbao-Castro JR, Robertson DL (2010) Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol 153:1398–1412
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500
Douzery EJ, Snell EA, Bapteste E, Delsuc F, Philippe H (2004) The timing of eukaryotic evolution: does a relaxed molecular clock reconcile proteins and fossils? Proc Natl Acad Sci USA 101:15386–15391
Feng HL, Ma NN, Meng X, Zhang S, Wang JR, Chai S, Meng QW (2013) A novel tomato MYC-type ICE1-like transcription factor, SIICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiol Biochem 73:309–320
Fisher F, Goding CR (1992) Single amino acid substitutions alter helix-loop-helix protein specificity for bases flanking the core CANNTG motif. EMBO J 11:4103–4109
Fu Z, Yu J, Cheng X, Zong X, Xu J, Chen M, Li Z, Zhang D, Liang W (2014) The rice basic helix-loop-helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development. Plant Cell 26:1512–1524
Gonzalez A, Zhao MZ, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827
Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC (2003) The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol 20:735–747
Jiang Y, Yang B, Deyholos MK (2009) Functional characterization of the Arabidopsis bHLH92 transcription factor in abiotic stress. Mol Genet Genomics 282:503–516
Jin JP, Zhang H, Kong L, Gao G, Luo JC (2014) PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res 42(D1):D1182–D1187
Kim DH, Yamaguchi S, Lim S, Oh E, Park J, Hanada A, Kamiya Y, Choi G (2008) SOMNUS, a CCCH type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20:1260–1277
Ledent V, Paquet O, Vervoort M (2002) Phylogenetic analysis of the human basic helix-loop-helix proteins. Genome Biol 3:RESEARCH0030
Li X, Duan X, Jiang H, Sun Y, Tang Y, Yuan Z, Guo J, Liang W, Chen L, Yin J, Ma H, Wang J, Zhang D (2006) Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol 141:1167–1184
Li F, Guo S, Zhao Y, Chen D, Chong K, Xu Y (2010) Overexpression of a homopeptide repeat-containing bHLH protein gene (OrbHLH001) from Dongxiang Wild Rice confers freezing and salt tolerance in transgenic Arabidopsis. Plant Cell Rep 29:977–986
Liu A, Wang Y, Zhang D, Wang X, Song H, Dang C, Yao Q, Chen K (2013) Classification and evolutionary analysis of the basic helix-loop-helix gene family in the green anole lizard, Anolis carolinensis. Mol Genet Genomics 288:365–380
Luby CH, Maeda HA, Goldman IL (2014) Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic Res 1:15
Massari ME, Murre C (2000) Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20:429–440
Murre C, McCaw PS, Baltimore D (1989) A new DNA binding and dimerizing motif in immunoglobulin enhancer binding, daugtherless, MyoD, and myc proteins. Cell 56:777–783
Peterson KJ, Lyons JB, Nowak KS, Takacs CM, Wargo MJ, McPeek MA (2004) Estimating metazoan divergence times with a molecular clock. Proc Natl Acad Sci USA 101:6536–6541
Pires N, Dolan L (2010) Origin and diversification of basic–helix-loop-helix proteins in plants. Mol Biol Evol 27:862–874
Schrader O, Ahne R, Fuchs J (2014) Karyotype analysis of Daucus carota L. using giemsa C-banding and FISH of 5S and 18S/25S rRNA specific genes. Caryologia 56:149–154
Simionato E, Ledent V, Richards G, Thomas-Chollier M, Kerner P, Coornaert D, Degnan BM, Vervoort M (2007) Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC Evol Biol 7:33
Soltis PS, Soltis DE (2004) The origin and diversification of angiosperms. Am J Bot 91:1614–1626
Song XM, Huang ZN, Duan WK, Ren J, Liu TK, Li Y, Hou XL (2014) Genome-wide analysis of the bHLH transcription factor family in Chinese cabbage (Brassica rapa ssp. pekinensis). Mol Genet Genomics 289:77–91
Stevens JD, Roalson EH, Skinner MK (2008) Phylogenetic and expression analysis of the basic helix-loop-helix transcription factor gene family: genomic approach to cellular differentiation. Differentiation 76:1006–1022
Tai HH, Goyer C, Murphy AM (2013) Potato MYB and bHLH transcription factors associated with anthocyanin intensity and common scab resistance. Botany 91:722–730
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tian C, Jiang Q, Wang F, Wang GL, Xu ZS, Xiong AS (2015) Selection of suitable reference genes for qPCR normalization under abiotic stresses and hormone stimuli in carrot leaves. PLOS One PONE-D-14-41274R2. doi:10.1371/journal.pone.0117569 [EMID:ac8290e9966e26d8]
Toledo-Ortiz G, Huq E, Quail PH (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15:1749–1770
Xu ZS, Chen M, Li LC, Ma YZ (2011) Functions and application of the AP2/ERF transcription factor family in crop improvement. J Integr Plant Biol 53:570–585
Xu ZS, Tan HW, Wang F, Hou XL, Xiong AS (2014) CarrotDB: a genomic and transcriptomic database for carrot. Database (Oxford) 2014. pii: bau096. doi: 10.1093/database/bau096
Yamaguchi SK, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Zhuang J, Cai B, Peng RH, Zhu B, Jin XF, Xue Y, Gao F, Fu XY, Tian YS, Zhao W, Qiao YS, Zhang Z, Xiong AS, Yao QH (2008) Genome-wide analysis of the AP2/ERF gene family in Populus trichocarpa. Biochem Biophys Res Commun 371:468–474
Zhuang J, Zhang J, Hou XL, Wang F, Xiong AS (2014) Transcriptomic, proteomic and functional genomic approaches for the study of abiotic stress in vegetable crops. Crit Rev Plant Sci 33:225–237
Acknowledgments
The research was supported by the New Century Excellent Talents in University (NCET-11-0670); Jiangsu Natural Science Foundation (BK20130027); China Postdoctoral Science Foundation (2014M551609), Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, YY., Li, MY., Wu, XJ. et al. Genome-wide analysis of basic helix−loop−helix family transcription factors and their role in responses to abiotic stress in carrot. Mol Breeding 35, 125 (2015). https://doi.org/10.1007/s11032-015-0319-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0319-0