Abstract
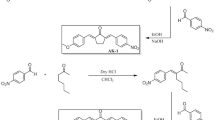
The design, synthesis, antinociceptive and β-adrenoceptor blocking activities of several eugenyloxy propanol azole derivatives have been described. In this synthesis, the reaction of eugenol with epichlorohydrin provided adducts 3 and 4 which were N-alkylated by diverse azoles to obtain the eugenyloxy propanol azole analogues in good yields. Adducts 3 and 4 were also reacted with azide ion to obtain the corresponding azide 6. The ‘Click’ Huisgen cycloaddition reaction of 6 with diverse alkynes afforded the title compounds in good yields. The synthesized eugenyloxy propanol azole derivatives were in vivo studied for the acute antinociception on male Spargue Dawley rats using tail-flick test. Compounds 5f, 5g, 7b and 11a exhibited potent analgesic properties in comparison with eugenol as a standard drug. In addition, all compounds were ex vivo tested for β-adrenoceptor blocking properties on isolated left atrium of male rats which exhibited partial antagonist or agonist behaviour compared to the standard drugs. The molecular docking study on the binding site of transient receptor potential vanilloid subtype 1 (TRPV1) has indicated that like capsaicin, eugenyloxy propanol azole analogues exhibited the strong affinity to bind at site of TPRV1 in a “tail-up, head-down” conformation and the presence of triazolyl moieties has played undeniable role in durable binding of these ligands to TRPV1. The in silico pharmacokinetic profile, drug likeness and toxicity predictions carried out for all compounds determined that 5g can be considered as potential antinociceptive drug candidate for future research.











Similar content being viewed by others
References
Nahin RL (2015) Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 16:769–780. https://doi.org/10.1016/j.jpain.2015.05.002
Pain. https://en.wikipedia.org/wiki/Pain. Accessed 6 Jan 2018
Kingston DGI (2011) Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod 74:496–511. https://doi.org/10.1021/np100550t
Bhuiyan MNI, Begum J, Chandra Nandi N, Akter F (2010) Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) alston). Afr J Plant Sci 4:451–454
Schmalz G, Arenholt-Bindslev D (2009) Biocompatibility of dental materials. Springer, Heidelberg
Yogalakshmi B, Viswanathan P, Anuradha CV (2010) Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 268:204–212. https://doi.org/10.1016/j.tox.2009.12.018
Rojo L, Vazquez B, Roman JS, Deb S (2008) Eugenol functionalized poly(acrylic acid) derivatives in the formation of glass-ionomer cements. Dent Mater 24:1709–1716. https://doi.org/10.1016/j.dental.2008.04.004
Schmidt E, Jirovetz L, Wlcek K, Buchbauer G, Gochev V, Girova T, Stoyanova A, Geissler M (2007) Antifungal activity of eugenol and various eugenol-containing essential oils against 38 clinical isolates of Candida albicans. J Essent Oil Bear Plants 10:421–429. https://doi.org/10.1080/0972060x.2007.10643575
Prakash P, Gupta N (2005) Therapeutic uses of Ocimum sanctum linn (tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol 49:125–131
Awasthi PK, Dixit SC, Dixit N, Sinha AK (2008) Eugenol derivatives as future potential drugs. J Pharm Res 1:215–220
Burt SA, Reinders RD (2003) Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol 36:162–167. https://doi.org/10.1046/j.1472-765x.2003.01285.x
Kim SI, Yi JH, Tak JH, Ahn YJ (2004) Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 120:297–304. https://doi.org/10.1016/j.vetpar.2003.12.016
Li Y, Xu C, Zhang Q, Liu JY, Tan RX (2005) In vitro anti-helicobacter pylori action of 30 chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol 98:329–333. https://doi.org/10.1016/j.jep.2005.01.020
Pisano M, Pagnan G, Loi M, Mura ME, Tilocca MG, Palmieri G, Fabbri D, Dettori MA, Delogu G, Ponzoni M, Rozzo C (2007) Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer 6(8):1–12. https://doi.org/10.1186/1476-4598-6-8
Zhao X, Chen D, Gao P, Ding P, Li K (2005) Synthesis of ibuprofen eugenol ester and its microemulsion formulation for parenteral delivery. Chem Pharm Bull 53:1246–1250. https://doi.org/10.1248/cpb.53.1246
Kaufman TS (2015) The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J Braz Chem Soc 26:1055–1085. https://doi.org/10.5935/0103-5053.20150086
Bendre RS, Rajput JD, Bagul SD, Karandikar PS (2016) Outlooks on medicinal properties of eugenol and its synthetic derivatives. Nat Prod Chem Res 4(3):100212–1000217. https://doi.org/10.4172/2329-6836.1000212
Fonseca-Berzal C, Ruiz FAR, Escario JA, Kouznetsov VV, Gómez-Barrio A (2014) In vitro phenotypic screening of 7-chloro-4-amino(oxy)quinoline derivatives as putative anti- Trypanosoma cruzi agents. Bioorg Med Chem Lett 24:1209–1213. https://doi.org/10.1016/j.bmcl.2013.12.071
Arango V, Domínguez JJ, Cardona W, Robledo SM, Muñoz SL, Figadere B, Sáez J (2012) Synthesis and leishmanicidal activity of quinoline–triclosan and quinoline–eugenol hybrids. Med Chem Res 21:3445–3454. https://doi.org/10.1007/s00044-011-9886-8
Chen H, Li G, Zhan P, Guo X, Ding Q, Wang S, Liu X (2013) Design, synthesis and biological evaluation of novel ligustrazinylated derivatives as potent cardiovascular agents. Med Chem Commun 4:827–832. https://doi.org/10.1039/c3md20352b
Hernández D, Bernal P, Cruz A, Garciafigueroa Y, Garduño L, Salazar M, Díaz F, Chamorro G, Tamariz J (2004) Potent hypolipidemic activity of mimetic amides of fibrates based on the 2-methoxy-4-(2-propenyl)phenoxyacetic scaffold. Drug Dev Res 61:19–36. https://doi.org/10.1002/ddr.10333
Huang YC, Wu BN, Yeh JL, Chen SJ, Liang JC, Lo YC, Chen IJ (2001) A new aspect of view in synthesizing new type beta-adrenoceptor blockers with ancillary antioxidant activities. Bioorg Med Chem 9:1739–1746. https://doi.org/10.1016/S0968-0896(01)00067-0
Bhat KI, Hussain MMM (2009) Synthesis, characterization and antimicrobial studies of some substituted pyrazolines from aryloxy acetyl hydrazine. Asian J Chem 21:3371–3375
Dallmeier K, Carlini EA (1981) Anesthetic, hypothermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogues. Pharmacology 22:113–127. https://doi.org/10.1159/000137479
Seixas Xavier FJ, da Franca Rodrigues KA, de Oliveira RG, Lima Junior CG, da Câmara RJ, Lima Keesen TS, de Oliveira MR, Lins Silva FP, de Almeida Vasconcellos MLA (2016) Synthesis and in vitro anti Leishmania amazonensis biological screening of Morita-Baylis-Hillman adducts prepared from eugenol, thymol and carvacrol. Molecules 21:1483. https://doi.org/10.3390/molecules21111483
Budavari S, O’Neil MJ, Smith A, Heckelman PE (2001) The merck index: an encyclopedia of chemicals, drugs, and biologicals, 13th edn. Merck & Co., Inc, Whitehouse Station
d’Avila Farias M, Oliveira PS, Dutra FSP, Fernandes TJ, de Pereira CMP, de Oliveira SQ, Stefanello FM, Lencina CL, Barschak AG (2013) Eugenol derivatives as potential anti-oxidants: is phenolic hydroxyl necessary to obtain an effect? J Pharm Pharmacol 66:733–746. https://doi.org/10.1111/jphp.12197
Abdul Rahim NHC, Asari A, Ismail N, Osman H (2017) Synthesis and antibacterial study of eugenol derivatives. Asian J Chem 29:22–26. https://doi.org/10.14233/ajchem.2017.20100
Chen SJ, Wang MH, Chen IJ (1996) Antiplatelet and calcium inhibitory properties of eugenol and sodium eugenol acetate. Gen Pharmacol 27:629–633. https://doi.org/10.1016/0306-3623(95)02089-6
Zhang P, Zhang E, Xiao M, Chen C, Xu W (2013) Enhanced chemical and biological activities of a newly biosynthesized eugenol glycoconjugate, eugenol α-d-glucopyranoside. Appl Microbiol Biotechnol 97:1043–1050. https://doi.org/10.1007/s00253-012-4351-2
Chen SJ, Huang YC, Wu BN, Chen IJ (1997) Eugenolol: AN eugenol-derived β-adrenoceptor blocker with partial β2-agonist and calcium mobilization inhibition associated vasorelaxant activities. Drug Dev Res 40:239–250. https://doi.org/10.1002/(sici)1098-2299(199703)40:3%3c239:aid-ddr4%3e3.0.co;2-l
A global brief on hypertension. http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf. Accessed 6 Jan 2017
Staessen JA, Gasowski J, Wang JG, Thijs L, Hond ED, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, Kerlikowske K, Pocock S, Fagard RH (2000) Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 355:865–872. https://doi.org/10.1016/s0140-6736(99)07330-4
Wilson CO, Gisvold O, Block JH (2004) Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry, 11th edn. Lippincott Williams & Wilkins, Philadelphia
Brunton LL, Lazo JS, Parker KL (2006) Goodman and Gilman’s the pharmacological basis of therapeutics, 11th edn. McGraw-Hill, New York
Patrick GL (2001) An introduction to medicinal chemistry, 2nd edn. Oxford University Press, New York
Gorre F, Vandekerckhove H (2010) Beta-blockers: focus on mechanism of action. Which beta-blocker, when and why? Acta Cardiol 65:565–570. https://doi.org/10.1080/ac.65.5.2056244
Kleeman A, Engel J, Kutscher B, Reichert D (1999) Pharmaceutical substances, 3rd edn. Thieme, Stuttgart
El Hadri A, Nicolle E, Leclerc G, Pietri-Rouxel F, Strosberg AD, Archimbault P (2003) New series of N-substituted phenyl ketone oxime ethers: synthesis and bovine β3-adrenergic agonistic activities. Pharmazie 58:13–17
Soltani Rad MN, Behrouz S, Dianat M (2008) Aqueous-mediated ring opening of epoxides with oximes: a rapid entry into β-hydroxy oxime O-ethers as potential β-adrenergic blocking agents. Synthesis 13:2055–2064. https://doi.org/10.1055/s-2008-1067122
Soltani Rad MN, Behrouz S, Karimitabar F, Khalafi-Nezhad A (2012) ‘Click synthesis’ of some novel O-substituted oximes containing 1,2,3-triazole-1,4-diyl residues as new analogs of β-adrenoceptor antagonists. Helv Chim Acta 95:491–501. https://doi.org/10.1002/hlca.201100324
Soltani Rad MN, Behrouz S, Doroodmand MM, Movahediyan A (2012) Copper-doped silica cuprous sulfate (CDSCS) as a highly efficient and new heterogeneous nano catalyst for [3+2] Huisgen cycloaddition. Tetrahedron 68:7812–7821. https://doi.org/10.1016/j.tet.2012.07.032
Soltani Rad MN, Behrouz S, Movahedian A, Doroodmand MM, Ghasemi Y, Rasoul-Amini S, Ahmadi Gandomani A-R, Rezaie R (2013) Doped nano-sized copper(I) oxide (Cu2O) on melamine-formaldehyde resin: a highly efficient heterogeneous nano catalyst for ‘Click’ synthesis of some novel 1H-1,2,3-triazole derivatives having antibacterial activity. Helv Chim Acta 96:688–701. https://doi.org/10.1002/hlca.201200224
Soltani Rad MN, Behrouz S, Zarenezhad E, Moslemin MH, Zarenezhad A, Mardkhoshnood M, Behrouz M, Rostami S (2014) Synthesis of fluorene and/or benzophenone O-oxime ethers containing amino acid residues and study of their cardiovascular and antibacterial effects. Med Chem Res 23:3810–3822. https://doi.org/10.1007/s00044-014-0967-3
Zhang Q, Ren H, Baker GL (2014) An economical and safe procedure to synthesize 2-hydroxy-4-pentynoic acid: a precursor towards ‘clickable’ biodegradable polylactide. Beilstein J Org Chem 10:1365–1371. https://doi.org/10.3762/bjoc.10.139
Asl MK, Nazariborun A, Hosseini M (2013) Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna J Phytomed 3:186–192
Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB (2009) Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain 144:84–94. https://doi.org/10.1016/j.pain.2009.03.016
Daniel AN, Sartoretto SM, Schmidt G, Caparroz-Assef SM, Bersani-Amado CA, Cuman RKN (2009) Anti-inflammatory and antinociceptive activities A of eugenol essential oil in experimental animal tests. Rev Bras Farmacogn 19:212–217. https://doi.org/10.1590/s0102-695x2009000200006
Tadiwos Y, Nedi T, Engidawork E (2017) Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum Hochst. ex. Dc. (Oleaceae) in mice. J Ethnopharmacol 202:281–289. https://doi.org/10.1016/j.jep.2017.02.036
Schunck RVA, Macedo IC, Laste G, de Souza A, Valle MTC, Salomon JLO, Nunes EA, Campos ACW, Gnoatto SCB, Bergold AM, Konrath EL, Dallegrave E, Arbo MD, Torres ILS, Leal MB (2017) Standardized Passiflora incarnata L. extract reverts the analgesia induced by alcohol withdrawal in rats. Phytother Res 31:1199–1208. https://doi.org/10.1002/ptr.5839
Huang YC, Wu BN, Lin YT, Chen SJ, Chiu CC, Cheng CJ, Chen IJ (1999) Eugenodilol: a third-generation beta-adrenoceptor blocker, derived from eugenol, with alpha-adrenoceptor blocking and beta2-adrenoceptor agonist-associated vasorelaxant activities. J Cardiovasc Pharmacol 34:10–20. https://doi.org/10.1097/00005344-199907000-00003
Jara-Oseguera A, Simon SA, Rosenbaum T (2008) TRPV1: on the road to pain relief. Curr Mol Pharmacol 1:255–269. https://doi.org/10.2174/1874-470210801030255
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543. https://doi.org/10.1016/s0896-6273(00)80564-4
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384. https://doi.org/10.1146/annurev-cellbio-101011-155833
Szallasi A, Blumberg PM (1990) Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root anglion membranes. Brain Res 524:106–111. https://doi.org/10.1016/0006-8993(90)90498-z
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284. https://doi.org/10.1016/j.cell.2009.09.028
Liao M, Cao E, Julius D, Cheng Y (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504:113–118. https://doi.org/10.1038/nature12823
Darré L, Domene C (2015) Binding of capsaicin to the TRPV1 ion channel. Mol Pharm 12:4454–4465. https://doi.org/10.1021/acs.molpharmaceut.5b00641
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49:3315–3321. https://doi.org/10.1021/jm051197e
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2009) Gaussian 09, Revision A.01 Gaussian, Inc., Wallingford CT
Bienstock RJ (2011) Library design, search methods, and applications of fragment-based drug design. American Chemical Society, Washington
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26. https://doi.org/10.1016/s0169-409x(00)00129-0
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today 1:337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Download DataWarrior V4.7.2. http://www.openmolecules.org/datawarrior/download.html. Accessed 2 Jan 2018
Acknowledgements
The authors wish to thank Shiraz University of Technology research council for partial support of this work. The authors are thankful from the High Performance Computing research laboratory of Institute for Research in Fundamental Sciences (IPM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Behrouz, S., Soltani Rad, M.N., Taghavi Shahraki, B. et al. Design, synthesis, and in silico studies of novel eugenyloxy propanol azole derivatives having potent antinociceptive activity and evaluation of their β-adrenoceptor blocking property. Mol Divers 23, 147–164 (2019). https://doi.org/10.1007/s11030-018-9867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9867-7