Abstract
The HIV-1 viral infectivity factor (VIF) protein is essential for viral replication. VIF recruits cellular ElonginB/C-Cullin5 E3 ubiquitin ligase to target the host antiviral protein APOBEC3G (A3G) for proteasomal degradation. Thus, the A3G-Vif–E3 complex represents an attractive target for the development of novel anti-HIV drugs. In this study, we describe the design and synthesis of indolizine derivatives as VIF inhibitors targeting the VIF–ElonginC interaction. Many of the synthesized compounds exhibited obvious inhibition activities of VIF-mediated A3G degradation, and 5 compounds showed improvement of activity compared to the known VIF inhibitor VEC-5 (1) with IC\(_{50 }\) values about 20 \(\upmu \)M. The findings described here will be useful for the development of more potent VIF inhibitors.
Similar content being viewed by others
Introduction
Since the first cases of AIDS were identified in 1981, remarkable medical advances have been made in the prevention and treatment of HIV/AIDS. The Food and Drug Administration (FDA) approved that anti-HIV drugs can be divided into six categories: nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleotide reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors (FIs), co-receptor inhibitors (CRIs), and integrase inhibitors (INIs) [1]. Combined application of these antiretroviral drugs has proven to be exceedingly effective at reducing viral load and improving the clinical status of many patients. However, viral resistance and toxicity associated with these inhibitors have created an urgent need to develop new anti-HIV drugs with novel targets and mechanisms [2–4]. As all currently approved anti-HIV drugs target HIV-1 Pol and Env-encoded proteins (i.e., reverse transcriptase (RT), protease (PR), and integrase (IN, gp41), widening the molecular targets of HIV therapy by additionally targeting regulatory and accessory proteins may lead to new effective therapies with expanded treatment options [5, 6].
Viral infectivity factor (VIF) is an HIV accessory protein, the primary function of which is to negate the action of APOBEC3G—a naturally occurring cellular inhibitor of HIV replication. In the absence of HIV-1 VIF, A3G is packaged into HIV-1 virions and introduces G-to-A hypermutations in viral minus-strand DNA during reverse transcription, which leads to the production of non-functional proviruses [7–10]. However, A3G-imposed replication block is primarily overcome by HIV-1 VIF that triggers the degradation of A3G by 26S proteasome through polyubiquitination. A normal cellular Elongin-Cullin-SOCS-box (ECS) ubiquitin ligase is comprised of ElonginB, ElonginC, Cullin5, Rbx2, and a cellular suppressor of cytokine signaling box (SOCS-box) protein. VIF targets A3G for polyubiquitination by mimicking SOCS-box protein that includes the BC-box, which binds ElonginB and ElonginC, and the Cullin box that has been proposed to participate in the binding of either Cullin5 or Cullin2 [11]. In this process, the N-terminal region of VIF binds to the N-terminal region of A3G, the zinc-binding region formed by a conserved HCCH motif interacts with Cullin5, and the BC-box region (residues 144 to 155) in the C-terminal of VIF binds to ElonginC [12–14]. Crystal structure of HIV VIF BC-box in complex with ElonginB and ElonginC shows more detailed interactions between side chains (Val142, Leu145, Leu148, Ala149, Ala152, and Leu153) of VIF residues and those of helix 3 and helix 4 of ElonginC, and the protein surface of ElonginC shows binding pockets for VIF Val142 and Leu145 [15]. Therefore, disrupting these binding interactions within the A3G-VIF–E3 complex presents new opportunities for therapeutic intervention. Several small molecule inhibitors of HIV-1 VIF (RN-18, IMB-26/35, and Baculiferins) have been identified (Figure 1) [16–19]. RN-18 and IMB-26/35 were identified by cell-based screening of chemical libraries, while Baculiferins were isolated from the marine sponge Iotrochota Baculifera. RN-18 was shown to inhibit VIF–A3G interaction through reduction of VIF in the host cells, and optimization of RN-18 has generated new inhibitors with improved activities [19]. IMB-26/35 analogs were shown to specifically bind to A3G, thereby stabilizing A3G expression and Baculiferins containing N-acetic acid group showed potent binding affinities toward both VIF and APOBEC3G.
To discover novel HIV-1 VIF inhibitors, our research was focused on identifying small molecule inhibitors of VIF–ElonginC interaction. A virtual screening was carried out based on ElonginC, and a small molecule 1 (VEC-5) with an IC\(_{50}\) of 36.1 \(\upmu \)M against HIV-1 VIF was obtained. Further biological investigation confirmed that VEC-5 protects A3G from VIF-mediated degradation through blocking interaction between VIF and ElonginC [20, 21]. Such a mechanism is different from reported HIV-1 VIF inhibitors and may lead to new approaches for anti-HIV-1 treatment.
Compound 1 is an indolizine derivative. Indolizine derivatives have been applied in drug discovery due to some unique properties of the scaffold [22, 23]. The biological activities of indolizines include antimicrobial, antioxidant, anti-inflammatory, anticonvulsant activities, etc. Many attractive methodologies of synthesis of indolizines have been developed. These methods could be classified as the Tschitschibabin reaction, 1, 3-dipolar cycloaddition, cyclisation reaction, etc., depending on the position of bond formation [22, 23]. For the optimization of compound 1, a series of indolizine derivatives have been synthesized in this study. The inhibition of VIF-mediated A3G-degradation and antiviral activities have been investigated. The determination of the structure-activity relationship (SAR) will be helpful for the discovery of more potent antiviral agents.
Results and discussion
Design
The binding model of 1 in complex with the crystal structure of human ElonginC (PDB code: 3DCG) was analyzed (Fig. 2). The model suggests that 1 largely mimics the conformation of a loop region of VIF BC-box. The naphthalene ring is stretched into the large hydrophobic cavity formed by Lys72, Val73, Tyr76, Pro94, Ile95, Pro97, Ile99, Pro97, Leu103, and Ala107, mimicking the interactions of Leu145 of VIF. The benzene ring of 1 is occupying another small hydrophobic groove mimicking the interactions of Val142 of VIF, while the ethyl moiety is extended outward from the ElonginC surface. There is no interaction between the indolizine ring and ElonginC and it was considered as a linker to provide proper angles and distances for the two aromatic moieties to stretch into the two hydrophobic pockets. Ester and 3-carbonyl moieties of 1 both act as hydrogen-bonding acceptors and form hydrogen bonds with Phe93 and Tyr79 of ElonginC, respectively. Overall, the most important interaction seems to be the \(\pi -\pi \) stacking between the naphthalene ring of 1 and Tyr76 of ElonginC.
Superposition of the predicted binding model of compound 1 in complex with ElonginC on the crystal structure of VIF BC-box in complex with ElonginC. The VIF BC-box and compound 1 are shown in blue and green, respectively, and the nitrogen and oxygen atoms in 1 are shown in blue and red, respectively. The ElonginC protein is shown in yellow, for clarity, only helices 3 and 4 of ElonginC are shown. Hydrogen bonds are shown in dashed light blue lines
With the desire to improve potency, modification of compound 1 was initially focused on the substituents at position 3 and 7. Aralkylamine was introduced to mimic the naphthalene as well as to reduce the rigidity of compounds 2. It was found that such replacement of naphthalene was tolerable; therefore, further modifications were based on compounds 2. Modification was further carried out by exchanging the 3-position and 7-position substituents of the indolizine core structure, which generated compounds 3 and 4. The length of the amide linker and the R substituent in compounds 3, as well as the acyl group in compounds 4 were investigated. The next modification was carried out by variation of the substitution pattern of the indolizine scaffold. The point of attachment of the the aroyl group in compounds 2 was moved from 7-position to 8-position of the indolizine core structure generated compounds 5, while the position of attachment of the 7-amide group in compound 4a was also hopped to 8-position of the indolizine ring-generated compound 6. The final modification was performed on the indolizine core structure by migrating the nitrogen atom position, which generated compound 7, or by insertion of one nitrogen atom to afford compound 8 (Fig. 3).
Chemistry
Compounds 2/5: These two series of analogs were synthesized by the following procedure (Scheme 1): Compounds 9 were reacted with benzyl 2-bromoacetate to afford pyridinium 10, then compounds 11 were obtained by constructing the indolizine ring through 1,3-dipolarcycloaddition of pyridinium 10 and ethyl propiolate [23, 24]. The benzyl-protecting group of compounds 11 was selectively removed using LiOH to afford the corresponding carboxylic acid intermediates 12, which were then condensed with various aryl alkylamines to afford analogs 2 and 5.
Reagents and conditions: a Benzyl 2-bromoacetate, ethyl acetate, reflux, overnight; b K\(_{2}\)CO\(_{3}\), CH\(_{3}\)CN, 0 \(^{\circ }\)C, 30 min, then ethyl propiolate, rt, 72 h; c LiOH, THF/C\(_{2}\)H\(_{5}\)OH/H\(_{2}\)O, rt, 2 h; d Ar\(_{2}\)CH\(_{2}\)NH\(_{2}\), EDC, HOBt, DIPEA, THF, rt, overnight
Compounds 3/4: 2-Bromo-1-phenylethanone was reacted with protected isonicotinic acids 13 to afford the corresponding pyridinium bromides 14, and then the indolizine ring was constructed by the same way mentioned above to afford 15. After deprotection, 16 was condensed with aralkylamines to afford analogs 3 and 4 (Scheme 2). The 3-aroyl was also replaced by several small ring amino acyl groups and the synthetic route was shown in Scheme 3. The 3, 7-bis-protected dicarboxylicacid indolizine 19 was synthesized by the same procedure as mentioned above. The tert-butyl- protecting group of compound 19 was first removed and the pyridin-2-yl-methanamine was introduced to afford compound 21. Then, the benzyl group was removed to get compound 22, which was then condensed with heterocyclic amines to afford compounds 4z.
Reagents and conditions: a Ar\(_{1}\)COCH\(_{2}\)Br, ethyl acetate, reflux, overnight; b K\(_{2}\)CO\(_{3}\), CH\(_{3}\)CN, 0 \(^{\circ }\)C, 30 min, then ethyl propiolate, rt, 72 h; c (1) R = tert-butyl: TFA, DCM, rt, overnight; (2) R = benzyl: TBAF, THF, rt, overnight; or LiOH, THF/C\(_{2}\)H\(_{5}\)OH/H\(_{2}\)O, rt, 2 h d EDC, HOBt, DIPEA, THF, rt, overnight.
Reagents and conditions: a Ethyl acetate, reflux, overnight; b Alkynyl acid acetic, K\(_{2}\)CO\(_{3}\), CH\(_{3}\)CN, 0 \(^{\circ }\)C-rt, 72 h; c TFA, DCM, rt, overnight; d Pyridin-2-yl-methanamine, EDC, HOBt, DIPEA, THF, rt, overnight; e LiOH, THF/EtOH/H\(_{2}\)O, rt, 2 h; f EDC, HOBt, DIPEA, R\(_{1}\)NR\(_{2}\), THF, rt, overnight
Compounds 6: The pyridinium bromide 24 was obtained by the reaction of 2-bromo-1-phenylethanone and tert-butyl nicotinate, which was subsequently reacted under typical indolizine construction conditions to afford 25. Then, the tert-butyl group was removed using TFA to afford the corresponding carboxylic acid 26, which was subsequently converted to the target compound 6 by condensing with pyridin-2-yl-methanamine (Scheme 4).
Compound 7: Compound 27 was reacted with 1-phenylprop-2-yn-1-one to afford the corresponding pyridinium bromide and then the indolizine core structure was constructed to give compound 29. The benzyl-protecting group of compound 29 was removed to afford the corresponding carboxylic acid 30. The target compound 7 was finally obtained by condensation of 30 with pyridin-2-yl-methanamine (Scheme 5).
Compound 8: The carboxyl group of compound 31 was protected as methyl ester to afford compound 32, which was then reacted with 1, 1-dimethoxy-\(N\), \(N\)-dimethylmethanamine to afford compound 33. The imidazo[1,2-a]pyridine core structure was constructed to afford 34 [25]. Deprotection of the ester group afforded compound 35, which was then condensed with pyridin-2-yl-methanamine to afford the target compound 8 (Scheme 6).
Biological activities and SARs
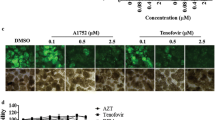
All the synthesized compounds were preliminarily evaluated for inhibiting VIF-mediated degradation of A3G effect at a single concentration of 50 \(\upmu \)M. The values are presented as the normalized intensity relative to the value in the HxB\(_{2}\)VIF-cmyc or untreated pEYFP-A3G group. The results are summarized in Table 1. Based on the preliminary biological results, 23 representative compounds were selected for anti-HIV-1 activity assay. The anti-HIV-1 activities were determined by a p24 antigen detection assay and the cytotoxicities of these compounds were evaluated by MTT assay. The results are summarized in Table 2.
Modification on the substituents at position 3 and 7
Compounds 2: In an effort to increase the solubility of the compounds, aralkylamines were introduced to mimic the \(\upbeta \)-naphthalene ring at position 3 of compound 1 as well as to reduce the rigidity of compounds 2. It was found that when the substituent at position 7 was maintained as unsubstituted aroyl, the replacement of \(\upbeta \)-naphthalene ring with aralkylamines (2a–2e) improved the activity of the compounds in the preliminary assay. The introduction of an electron-donating group (2f, 2g) to the aroyl led to reduction in potency, while an electron-withdrawing group gave variable results. Compound 2h showed reduced activity, while 2i showed improved activity in the preliminary assay. Further, anti-HIV-1 activity assay showed that compounds 2d and 2e exhibited slightly reduced activity compared to compound 1, which suggested that the replacement of the \(\upbeta \)-naphthalene ring by aralkylamines is toleratable to some extent.
Compounds 3/4: In these two series of compounds, modifications were made to substituents at position 3 and7 simultaneously based on the hypothesis that the terminal aryl groups of the 3,7-substituents were critical for interaction with target, and the potency can be retained as long as the distance and orientation of the two aryl groups were retained. Therefore, compounds 3/4 were obtained by exchanging the substituents at position 3 and 7.
Compounds 3 were designed to investigate the effect of aralkylamine for activity in detail, while the aroyl at position 3 was maintained as benzoyl group. Various types of aralkylamine were introduced and the generated compounds showed similar to improved activities when \(n=0\) (3a–3k). The introduction of R substituents showed no adverse effect on activity considering the chirality of the substituent (3f–3h). However, the activity of compounds decreased when \(n=1\) (3l–3n), with the exception of compound 3o, which indicated that the 3-atom separation between the Ar and the indolizine ring was favorable for activity. The same trend was observed in the anti-HIV-1 activity, 6 of the compounds bearing 3-atom separation between the Ar and the indolizine ring showed an IC\(_{50}\) of about 50 \(\upmu \)M while the 4-atom separation compound 3o showed an IC\(_{50}\) of 103 \(\upmu \)M. The most potent compounds in this series were 3c and 3j with IC\(_{50 }\) values of 19.3 and 20.1 \(\upmu \)M, respectively, and both showed low cytotoxicities.
Compounds 4 were designed to investigate the effect of 3-position aroyl on activity when pyridin-2-yl-methanamine was maintained at position 7. Various substitutents were introduced to the phenyl ring of the benzoyl group to identify the optimal substituent. In general, introduction of an electron-withdrawing group (Cl, Br, CF\(_{3}\) and NO\(_{2}\)) to the ortho-position of the phenyl ring decreased activity, while the introduction of an electron-donating (4f) group improved activity. Compound 4f also showed comparable anti-HIV-1 activity (IC\(_{50 }\)= 45.5 \(\upmu \)M) compared to 3a (IC\(_{50 }\)= 47.0 \(\upmu \)M). The introduction of substituents to the meta-position of the phenyl ring resulted in compounds with similar to improved activities with the exception of compound 4k. The meta-amine compound 4l (IC\(_{50 }\)= 22.3 \(\upmu \)M) exhibited improved anti-HIV-1 activity and lower toxicity compared to compound 1, while the bromine at the meta-position (4i) increased cytotoxicity. Furthermore, neither the introduction of an electron-withdrawing group nor an electron-donating group provided similar to improved activity. The anti-HIV-1 activity of the para-substituted compounds correlated well with the electron density of the aryl ring, the poorer electron density of the aryl ring, the higher activity the compound showed (NO\(_{2 }\approx \) CF\(_{3 }>\) F \(>\) Cl \(>\) NH\(_{2})\). Compounds 4p and 4q both showed improved activity compared to 1 with IC\(_{50}\) of 20.5 \(\upmu \)M. Introduction of two substituents to the phenyl ring decreased the antiviral activity in general except for compound 4v. But, the anti-HIV-1 activity of compound 4v decreased significantly compared to 1. The compounds were tested with further modification of the phenyl group into its bioisosteres, such as furan and thiophene. The resulted compounds showed comparable activities to 3a, but did not show improved potency compared to compound 1. Introduction of large aryl group (4y) was also disfavor for activity.
The 3-position aroyl was further modified by replacing the phenyl ring with saturated heterocycles (4z). This group only produced one active compound (4z–2), but compound 4z–2 had weak anti-HIV-1 activity.
Variation of the substitution pattern of the indolizine scaffold
Compounds 5/6: The substituent at position 7 of compounds 2 was moved to position 8, while the 3-position substituent was maintained as amide group which genenated compounds 5. The 7-position amide group of compound 3a was also investigated by changing the substitution site to the 8-position of the indolizine ring which generated compound 6. The resulted compounds 5 showed significant decrease activities, while compound 6 showed increased activity in the preliminary assay. However, 6 showed about onefold decrease of anti-HIV-1 activity compared to compound 1 suggesting that the position of the aroyl group is important for activity.
Replacement of the indolizine moiety
Compounds 7/8: Finally, two mimics of the indolizine core structure were synthesized and evaluated in the preliminary assay. When the N atom of the indolizine was migrated, the resulted compound 7 showed comparable activity. Then, an additional N atom was incorporated to the indolizine ring, which resulted in the imidazo[1, 2-a]pyridine core structure compound 8 with the carbethoxy group being elimated. The activity of compound 8 decreased compared to 1, which suggested that the carbethoxy group may play an important role in maintaining activity.
General conclusions about the SAR
Compounds 2 demonstrated that the naphthalene ring can be replaced by aralkylamines. The exchange of substituents at position 3 and 7 was well tolerated as shown in compounds 3 and 4. Also, the substitution pattern of the indolizine scaffold was important, moved the 7-position substituent to the 8-position resulted in decrease in activity as shown in compounds 5 and 6. The isosteric replacement of the indolizine moiety was also tolerated, as long as the substituents were retained.
Molecular docking: To elucidate the binding model of compounds with ElonginC, three representative compounds (1, 2a, and 3a) were selected for docking simulation studies.
Superimposition of the docked conformations of compounds 1, 2a, and 3a to ElonginC shows the similarities of their bindings (Fig. 4). Both of the aryl rings of the aralkylamine group in compounds 2a and 3a are almost parallel to Tyr76 and form \(\pi -\pi \) stacking and hydrophobic interactions which are similar to compound 1’s naphthalene ring. The aroyl groups of both compounds are located in the small hydrophobic pocket where compound 1’s benzoyl group was located. The ethoxycarbonyl moiety of both compounds is extended outward from the ElonginC surface just like compound 1. These interactions were thought to be crucial for ligands to bind to the receptor, which may be the reasons for these derivatives to maintain activity.
Superimposition of binding modes of compounds 1, 2a, and 3a docked into the binding site of ElonginC. The carbon atoms of compounds 1, 2a, and 3a are shown in green, orange, and cyan, respectively, and the nitrogen and oxygen atoms in all ligands are shown in blue and red, respectively. Hydrogen-bonding interaction between ligand and residues are shown with blue dotted lines
Conclusions
This study has identified new amide indolizine derivatives as HIV-1 VIF inhibitors based on the lead compound 1. Studies were directed mainly at finding better replacement and suitable substitution site of the amide functionality present in the novel amide indolizine derivatives. The aroyl group was also investigated in an effort to improve anti-HIV-1 activity. Among all the 62 synthesized derivatives of compound 1, 5 compounds with the 3-aroyl, 7-amide indolizine structure showed improvement of activity compared to 1 with IC\(_{50 }\) values about 20 \(\upmu \)M. Overall, these indolizine derivatives show potential as effective HIV-1 VIF–ElonginC interaction inhibitors and the detailed study for structure-activity relationship may lead us to find more potent inhibitors.
Experimental section
Chemistry
All chemicals and solvents were purchased from commercial suppliers of analytical grade and used without further purification unless otherwise indicated. \(^{1}\)H NMR and \(^{13}\)C NMR spectra were recorded at 400 and 100 MHz, respectively, on a Bruker Avance III 400 NMR spectrometer. Chemical shifts are reported as values from an internal tetramethylsilane standard. High-resolution mass spectra (HRMS) were recorded on a Bruker Apex IV FTMS. LC/MS analyses were performed on an Aglient 1200-6110 instruments. Elemental analyses were performed on a Vario EL III instrument and are \(\pm \)0.3 of theoretical. Melting points were determined on a MelTemp apparatus and were uncorrected.
General procedure for the synthesis of compounds 2/5
A mixture of 4-aroyl-pyridin 9 (10 mmol) and benzyl 2-bromoacetate (12 mmol) in THF was heated to reflux overnight. The solvent was evaporated and the residue was used for the next reaction without further purification.
The pyridinium salt 10 (10 mmol) was suspended in CH\(_{3}\)CN (60 mL) at 0 \(^{\circ }\)C and then potassium carbonate (20 mmol) was added portionwise to maintain the temperature at 0 \(^{\circ }\)C. After 30 min, ethyl propiolate (11 mmol) was added dropwise at 0 \(^{\circ }\)C, and the resulting mixture was stirred at room temperature for 3 days. The solvent was evaporated under reduced pressure and the residue was diluted with water, extracted three times with CH\(_{2}\)Cl\(_{2}\). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, and evaporated. The crude product was purified by silica gel chromatography to afford 11.
LiOH\(\cdot \)H\(_{2}\)O (2 mmol) was added to the solution of compound 11 (1 mmol) in THF (10 mL), EtOH (10 mL) and H\(_{2}\)O (3 mL). The reaction mixture was stirred at room temperature overnight. The solvent was evaporated under reduced pressure and 1N HCl solution was added to the residue. The precipitated product was filtered and washed with water to afford 12. The crude product was used for the next reaction without further purification or characterization.
The crude carboxylic acid 12 (0.3 mmol) was dissolved in THF (15 mL), then EDC (0.45 mmol), HOBt (0.45 mmol), DIPEA (0.6 mmol), and aralkylamine (0.36 mmol) were added. The reaction mixture was stirred overnight at room temperature. After the solvent was removed, the residue was extracted with CH\(_{2}\)Cl\(_{2}\). The combined organic extracts were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was concentrated and the residue was purified by silica gel chromatography to afford 2.
Ethyl 7-picolinoyl-3-(pyridin-2-ylmethylcarbamoyl) indolizine-1-carboxylate (2a)
Yellow solid; Yield: 65.5 %; Mp: 165–167 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.75 (d, \(J\) = 6.1 Hz, 1H, indolizine-H-5), 9.20 (s, 1H, indolizine-H-2), 8.78 (s, 1H, ArH), 8.61 (s, 1H, ArH), 8.11 (d, \(J\) = 7.3 Hz, 1H, ArH), 8.00–7.85 (m, 2H, ArH), 7.77–7.67 (t, \(J\) = 7.0 Hz, 1H, ArH), 7.66–7.47 (m, 3H, ArH), 7.37 (d, \(J\) = 7.3 Hz, 1H, ArH), 7.30–7.25 (s, 1H, NH), 4.79 (d, \(J\) = 5.1 Hz, 2H, Ar-CH\(_{2}\)), 4.38 (q, \(J\) = 6.0 Hz, 2H, CH\(_{2}\)), 1.38(t, \(J\) = 6.0 Hz, 3H, CH\(_{3}\)); \(^{13}\)C NMR (101 MHz, CDCl\(_{3}\)) \(\delta \) 191.24, 164.11, 161.25, 156.10, 154.83, 149.21, 148.77, 137.47, 137.25, 136.23, 131.30, 127.85, 126.73, 125.34, 124.98, 122.81, 122.41, 120.19, 119.42, 113.51, 109.11, 60.47, 44.34, 14.66; ESI-MS m/z: 429.0 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+}\)) m/z Calcd. for C\(_{24}\)H\(_{20}\)N\(_{4}\)O\(_{4}\) [M+H]\(^{+}\): 429.1485; Found: 429.1553.
Ethyl 7-picolinoyl-3-(pyridin-3-ylmethylcarbamoyl) indolizine-1-carboxylate (2b)
Yellow solid; Yield: 78.1 %; Mp: 135–137 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.72 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 9.18 (s, 1H, indolizine-H-2), 8.76 (d, \(J\) = 3.0 Hz, 1H, ArH), 8.63 (s, 1H, ArH), 8.54 (d, \(J\) = 2.9 Hz, 1H, ArH), 8.11 (d, \(J\) = 7.8 Hz, 1H), 7.95 (t, \(J\) = 7.7 Hz, 1H), 7.81 (s, 1H, indolizine-H-8), 7.75 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.61 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.54 (t, \(J\) = 5.9 Hz, 1H, ArH), 7.30 (t, \(J\) = 5.5 Hz, 1H, ArH), 6.90 (t, \(J\) = 4.3 Hz, 1H, NH), 4.69 (d, \(J\) = 5.1 Hz, 2H, Ar-CH\(_{2})\), 4.33 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.34 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 191.2, 164.0, 161.3, 154.7, 149.5, 149.3, 148.7, 137.5, 136.4, 135.9, 134.1, 131.6, 127.8, 126.8, 125.2, 125.0, 123.9, 120.1, 118.9, 113.7, 109.0, 60.5, 41.2, 14.6; ESI-MS m/z: 429.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{24}\)H\(_{20}\)N\(_{4}\)O\(_{4}\): C, 67.00; H, 4.69; N, 12.93; Found: C, 67.28; H, 4.71; N, 13.08.
Ethyl 3-(benzylcarbamoyl)-7-picolinoylindolizine -1-carboxylate (2c)
Yellow solid; Yield: 78.1 %; Mp: 170–173 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.74 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 9.18 (s, 1H, indolizine-H-2), 8.77 (d, \(J\) = 3.3 Hz, 1H, ArH), 8.10 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.94 (t, \(J\) = 7.8 Hz, 1H, ArH), 7.74 (s, 1H, indolizine-H-8), 7.60 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6), 7.56-7.48 (m, 1H, ArH), 7.41–7.28 (m, 5H, ArH), 6.45 (t, \(J\) = 4.3 Hz,1H, NH), 4.66 (d, \(J\) = 4.9 Hz, 2H, Ar-CH\(_{2})\), 4.33 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.34 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 191.2, 164.0, 161.1, 154.8, 148.7, 138.2, 137.5, 136.3, 131.4, 129.1, 128.1, 127.9, 127.9, 126.8, 125.3, 125.0, 119.7, 119.2, 113.6, 109.0, 60.5, 43.7, 14.6; ESI-MS m/z: 428.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{4}\): C, 70.09; H, 4.94; N, 9.76; Found: C, 70.25; H, 4.95; N, 9.83.
Ethyl 7-(furan-2-carbonyl)-3-(pyridin- 2-yl-methylcarbamoyl)indolizine-1-carboxylate (2d)
Yellow solid; Yield: 69.3 %; Mp: 166–169 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.75 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 9.10 (s, 1H, indolizine-H-2), 8.61 (d, \(J\) = 4.4 Hz, 1H, ArH), 7.91 (s, 1H, ArH), 7.76 (s, 1H, ArH), 7.73 (t, \(J\) = 7.8 Hz, 1H, ArH), 7.69–7.62 (m, 1H, NH), 7.49 (d, \(J\) = 7.5 Hz, 1H, indolizine-H-6), 7.40 (d, \(J\) = 2.5 Hz, 1H, ArH), 7.37 (d, \(J\) = 7.9 Hz, 1H, ArH), 7.26 (d, \(J\) = 7.7 Hz, 2H, ArH), 6.65 (s, 1H, ArH), 4.79 (d, \(J\) = 4.4 Hz, 2H, ArCH\(_{2})\), 4.42 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 179.8, 164.1, 161.2, 156.1, 152.4, 149.2, 147.5, 137.2, 136.3, 132.1, 128.2, 122.8, 122.5, 120.8, 120.1, 119.4, 112.9, 112.7, 108.6, 60.5, 44.3, 14.7; ESI-MS m/z: 418.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{23}\)H\(_{19}\)N\(_{3}\)O\(_{5}\): C, 66.18; H, 4.59; N, 10.07; Found: C, 66.10; H, 4.70; N, 10.04.
Ethyl 7-(furan-2-carbonyl)-3-(pyridin- 3-yl-methylcarbamoyl)indolizine-1-carboxylate (2e)
Yellow solid; Yield: 93.3 %; Mp: 134–136 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.72 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 9.05 (s, 1H, indolizine-H-2), 8.63 (s, 1H, ArH), 8.53 (s, 1H, ArH), 7.86 (s, 1H, ArH), 7.80–7.71(m, 2H, ArH+NH), 7.48 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6), 7.42–7.36 (m, 1H, ArH), 7.28 (s, 1H, ArH), 7.18 (t, \(J\) = 4.6 Hz, 1H, ArH), 6.65 (s, 1H, ArH), 4.69 (d, \(J\) = 5.5 Hz, 2H, ArH), 4.35 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.38 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 179.7, 164.0, 161.3, 152.3, 149.4, 149.1, 147.5, 136.4, 136.0, 132.3, 128.2, 123.9, 122.4, 120.9, 120.1, 119.0, 113.0, 112.7, 108.6, 60.6, 41.1, 14.6; ESI-MS m/z: 418.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{23}\)H\(_{19}\)N\(_{3}\)O\(_{5}\): C, 65.92; H, 4.62; N, 10.10; Found: C, 66.18; H, 4.59; N, 10.07.
Ethyl 7-(3-methoxybenzoyl)-3-(pyridin-2-yl methylcarbamoyl)indolizine-1-carboxylate (2f)
Yellow solid; Yield: 54.6 %; Mp: 152–153 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.77 (d, \(J\) = 7.5 Hz, 1H, indolizine-H-5), 8.69 (s, 1H, indolizine-H-2), 8.61 (s, 1H, ArH), 7.91 (s, 1H, indolizine-H-8), 7.75–7.69 (t, \(J\) = 7.6 Hz, 1H, ArH), 7.68-7.60 (br, 1H, NH), 7.45–7.33 (m, 3H, ArH), 7.36 (d, \(J\) = 7.6 Hz, 1H, indolizine-H-6), 7.25 (d, \(J\) = 7.0 Hz, 1H, ArH), 7.16 (d, \(J\) = 7.0 Hz, 1H, ArH), 4.79 (d, \(J\) = 4.4 Hz, 2H, ArCH\(_{2})\), 4.34 (q, \(J\) = 6.8 Hz, 2H, CH\(_{2})\), 3.87 (s, 3H, OCH\(_{3})\), 1.33 (t, \(J\) = 6.9 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 194.3, 164.0, 161.2, 159.9, 156.1, 149.3, 138.4, 137.1, 136.1, 132.5, 129.6, 128.2, 123.5, 122.7, 122.3, 120.1, 119.4, 114.3, 113.3, 108.6, 60.5, 55.7, 44.4, 14.5; ESI-MS m/z: 458.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{23}\)N\(_{3}\)O\(_{5}\): C, 68.16; H, 5.12; N, 9.17; Found: C, 68.26; H, 5.07; N, 9.19.
Ethyl 7-(3-methoxybenzoyl)-3-(pyridin-3-yl methylcarbamoyl)indolizine-1-carboxylate (2g)
Yellow solid; Yield: 43.8 %; Mp: 128–130 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.74 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.65 (s, 1H, indolizine-H-2), 8.61 (s, 1H, ArH), 8.53–8.48 (m, 1H, NH), 7.90 (s, 1H, indolizine-H-8), 7.75 (d, \(J\) = 7.5 Hz, 1H, ArH), 7.46–7.34 (m, 5H, ArH + indolizine-H-6), 7.27 (t, \(J\) = 6.3 Hz, 1H, ArH), 7.16 (d, \(J\) = 7.5 Hz, 1H, ArH), 4.68 (d, \(J\) = 5.3 Hz, 2H, ArCH\(_{2})\), 4.27 (q, \(J\) = 6.7 Hz, 2H, CH\(_{2})\), 3.86 (s, 3H, OCH\(_{3})\), 1.26 (t, \(J\) = 6.7 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 194.3, 164.0, 161.4, 159.9, 149.4, 149.1, 138.3, 136.2, 135.9, 134.3, 132.7, 129.6, 128.1, 123.9, 123.4, 122.7, 120.3, 119.4, 119.1, 114.4, 113.4, 108.5, 60.5, 55.6, 41.1, 14.4; ESI-MS m/z: 458.0 [M+H]\(^{+}\).
Ethyl 7-(2-chloroisonicotinoyl)-3-(pyridin-2-yl methylcarbamoyl)indolizine-1-carboxylate (2h)
Yellow solid; Yield: 50.1 %; Mp: 187–190 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.79 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.67–8.59 (m, 3H, ArH), 7.92 (s, 1H, indolizine-H-2), 7.79–7.69 (m, 2H, ArH+NH), 7.68 (s, 1H, indolizine-H-8), 7.56 (d, \(J\) = 5.0 Hz, 1H, ArH), 7.42 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6, 7.36 (d, \(J\) = 7.5 Hz, 1H, ArH), 7.30–7.25 (m, 1H, ArH), 4.79 (d, \(J\) = 4.5 Hz, 2H, Ar-CH\(_{2})\), 4.35 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.34 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 191.5, 163.7, 161.0, 155.9, 152.6, 150.6, 149.3, 147.1, 137.2, 135.5, 130.1, 128.6, 124.3, 123.8, 122.8, 122.4, 121.6, 120.3, 120.2, 112.0, 109.8, 60.7, 44.4, 14.5; ESI-MS m/z: 463.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{24}\)H\(_{19}\)ClN\(_{4}\)O\(_{4}\): C, 62.01; H, 4.22; N, 11.95; Found: C, 62.27; H, 4.14; N, 12.10.
Ethyl 7-(2-chloroisonicotinoyl)-3-(pyridin-3-yl methylcarbamoyl)indolizine-1-carboxylate (2i)
Yellow solid; Yield: 9.4 %; Mp: 189–192 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.78 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.67–8.58 (m, 3H, ArH), 8.55 (s, 1H, ArH), 7.85 (s, 1H, indolizine-H-2), 7.76 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.67 (s, 1H, indolizine-H-8), 7.56 (d, \(J\) = 4.7 Hz, 1H, ArH), 7.45 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6), 7.32 (t, \(J\) = 4.9 Hz, 1H, ArH), 6.99 (t, \(J\) = 5.3 Hz, 1H, NH), 4.70 (d, \(J\) = 5.3 Hz, 2H, Ar-CH\(_{2})\), 4.32 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.31 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 191.4, 163.6, 161.1, 152.7, 150.7, 149.5, 149.3, 146.9, 136.0, 135.7, 134.0, 130.4, 128.7, 124.3, 124.0, 123.8, 121.5, 120.3, 119.7, 112.3, 109.8, 60.8, 41.2, 14.5; ESI-MS m/z: 463.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{24}\)H\(_{19}\)ClN\(_{4}\)O\(_{4}\): C, 62.01; H, 4.22; N, 11.95; Found: C, 62.27; H, 4.14; N, 12.10.
Ethyl 8-benzoyl-3-(pyridin-2-yl methylcarbamoyl)indolizine-1-carboxylate (5a)
Off white solid; Yield: 90.9 % over two steps; Mp: 173–175 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.91 (d, \(J\) = 7.0 Hz, 1H, indolizine-H-5), 8.60 (s, 1H, indolizine-H-2), 7.92–7.80 (m, 3H, ArH), 7.71 (t, \(J\) = 7.6 Hz, 1H, ArH), 7.65–7.59 (m, 1H, NH), 7.56 (t, \(J\) = 6.53 Hz,1H, ArH), 7.43 (t, \(J\) = 7.3 Hz, 2H, ArH), 7.36 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.26–7.17 (m, 2H, ArH + indolizine-H-7), 6.97 (t, \(J\) = 6.9 Hz, 1H, indolizine-H-6), 4.79 (s, 2H, Ar-CH\(_{2})\), 4.00 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.02 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (101 MHz, CDCl\(_{3}) \delta \) 193.49, 163.53, 161.49, 156.27, 149.19, 137.39, 137.16, 133.74, 133.31, 131.70, 129.90, 129.85, 128.63, 125.85, 122.72, 122.25, 120.37, 118.24, 112.97, 106.51, 60.12, 44.35, 14.27; ESI-MS m/z: 428.1 [M+H]\(^{+}\).
Ethyl 8-benzoyl-3-(pyridin-3-yl methylcarbamoyl)indolizine-1-carboxylate (5b)
Yellowish green pale solid; Yield: 93.7 %; Mp: 196–196 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.86 (d, \(J\) = 6.8 Hz, 1H, indolizine-H-5), 8.73–8.36 (m, 2H, ArH), 7.90 (s, 1H, indolizine-H-2), 7.84 (d, \(J\) = 7.4 Hz, 2H, ArH), 7.74 (d, \(J\) = 7.0 Hz, 2H, ArH), 7.55 (t, \(J\) = 7.0 Hz, 1H, ArH), 7.42 (t, \(J\) = 7.3 Hz, 2H, ArH), 7.25 (br, 1H, NH), 7.21 (d, \(J\) = 6.7 Hz, 1H, indolizine-H-7), 6.95 (t, \(J\) = 6.7 Hz, 1H, indolizine-H-6), 4.64 (s, 2H, Ar-CH\(_{2})\), 3.87 (q, \(J\) = 6.4 Hz, 2H, CH\(_{2})\), 0.89 (t, \(J\) = 6.7 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 193.6, 163.6, 161.6, 148.9, 148.4, 137.1, 135.9, 133.7, 133.4, 131.5, 129.8, 129.8, 128.6, 125.9, 120.7, 118.0, 112.9, 106.3, 60.0, 40.9, 14.0; ESI-MS m/z: 428.0 [M+H]\(^{+}\), 854.8 [2M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{4}\): C, 70.07; H, 4.96; N, 9.74; Found: C, 70.25; H, 4.95; N, 9.83.
Ethyl 8-picolinoyl-3-(pyridin-2-yl methylcarbamoyl)indolizine-1-carboxylate (5c)
Yellow solid; Yield: 15.9 %; Mp: 64–66 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.91 (d, \(J\) = 6.9 Hz, 1H, indolizine-H-5), 8.60 (s, 1H, ArH), 8.51 (s, 1H, ArH), 8.29 (d, \(J\) = 7.5 Hz, 1H, ArH), 7.90 (t, \(J\) = 7.4 Hz, 1H, ArH), 7.81 (s, 1H, indolizine-H-2), 7.71 (t, \(J\) = 7.6 Hz, 1H,), 7.53 (s, 1H, NH), 7.42 (t, \(J\) = 4.8 Hz, 1H, ArH), 7.39–7.31 (m, 2H, ArH), 7.24 (t, \(J\) = 4.8 Hz, 1H, ArH), 7.02 (t, \(J\) = 6.8 Hz, 1H, indolizine-H-6), 4.78 (d, \(J\) = 3.9 Hz, 2H, Ar-CH\(_{2})\), 3.97 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.07 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 193.3, 163.8, 161.6, 156.5, 154.7, 149.3, 149.0, 137.0, 134.7, 131.8, 130.0, 126.8, 126.7, 126.4, 123.2, 122.6, 122.2, 119.9, 117.9, 113.2, 106.2, 60.0, 44.4, 14.4; ESI-MS m/z: 429.1 [M+H]\(^{+}\), 879.1 [2M+Na]\(^{+}\).
Ethyl 8-picolinoyl-3-(pyridin-3-yl methylcarbamoyl)indolizine-1-carboxylate (5d)
Yellow solid; Yield: 60.7 %; Mp: 158–160 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.86 (d, \(J\) = 7.0 Hz, 1H, indolizine-H-5), 8.53 (s, 1H, ArH), 8.51–8.42 (m, 2H, ArH), 8.27 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.89 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.73–7.66 (m, 2H, NH+indolizine-H-2), 7.41 (t, \(J\) = 5.1 Hz,1H, ArH), 7.34 (d, \(J\) = 6.9 Hz, 1H indolizine-H-7), 7.28–7.21 (m, 2H, ArH), 7.01 (t, \(J\) = 6.9 Hz, 1H, indolizine-H-6), 4.58 (d, \(J\) = 5.1 Hz, 2H, Ar-CH\(_{2})\), 3.87 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 0.97 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 193.3, 163.6, 161.6, 154.6, 149.5, 149.4, 149.3, 148.9, 148.8, 137.1, 136.0, 135.8, 134.7, 134.5, 132.4, 131.8, 130.0, 126.8, 126.5, 123.9, 123.8, 123.3, 120.1, 117.5, 113.3, 106.1, 59.9, 40.9, 14.2; ESI-MS m/z: 429.0 [M+H]\(^{+}\), 857.0 [2M+H]\(^{+}\).
Ethyl 8-(furan-2-carbonyl)-3-(pyridin-2-yl methylcarbamoyl)indolizine-1-carboxylate (5e)
Yellow solid; Yield: 100 %; Mp: 179-181 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \quad \delta \) 9.92 (d, \(J\) = 7.0 Hz, 1H, indolizine-H-5), 8.61 (s, 1H, ArH), 7.87 (s, 1H, indolizine-H-2), 7.71 (t, \(J\) = 7.5 Hz, 1H, ArH), 7.63 (s, 1H, ArH), 7.53 (s, 1H, NH), 7.36 (t, \(J\) = 8.3 Hz, 2H, ArH), 7.24 (t, \(J\) = 6.9 Hz, 1H, indolizine-H-6), 7.03–6.94 (m, 2H, ArH), 6.53 (s, 1H, ArH), 4.78 (d, \(J\) = 3.2 Hz, 2H, Ar-CH\(_{2})\), 4.12 (q, \(J\) = 6.8 Hz, 2H, CH\(_{2})\), 1.17 (t, \(J\) = 6.7 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \quad \delta \) 181.1, 163.7, 161.4, 156.2, 152.9, 149.3, 147.3, 137.1, 133.5, 130.6, 130.4, 126.2, 122.7, 122.2, 120.4, 119.4, 118.3, 112.9, 112.5, 106.5, 60.4, 44.4, 14.3; ESI-MS m/z: 418.0 [M+H]\(^{+}\).
Ethyl 8-(furan-2-carbonyl)-3-(pyridin-3-yl methylcarbamoyl)indolizine-1-carboxylate (5f)
Yellow solid; Yield: 96.2 %; Mp: 189–191 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.87 (d, \(J\) = 6.9 Hz, 1H, indolizine-H-5), 8.60 (s, 1H, indolizine-H-2), 8.49 (s, 1H, ArH), 7.86 (s, 1H, ArH), 7.73 (d, \(J\) = 7.5 Hz, 1H, ArH), 7.63 (s, 1H, ArH), 7.45 (s, 1H, ArH), 7.36 (d, \(J\) = 6.8 Hz, 1H, indolizine-H-7), 7.26 (t, \(J\) = 5.2 Hz, 1H, ArH), 7.01 (s, 1H, ArH), 6.96 (t, \(J\) = 6.9 Hz, 1H, indolizine-H-6), 6.52 (s, 1H, ArH), 4.66 (d, \(J\) = 5.4 Hz, 2H, Ar-CH\(_{2})\), 4.00 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.05 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 181.0, 163.7, 161.6, 152.7, 149.3, 148.9, 147.4, 135.8, 134.4, 133.4, 130.4, 130.3, 126.3, 123.8, 120.6, 119.7, 117.9, 112.9, 112.5, 106.4, 60.3, 41.0, 14.1; ESI-MS m/z: 418.0 [M+H]\(^{+}\), 835.0 [2M+H]\(^{+}\).
General procedure for the synthesis of compounds 3/4
A mixture of Ar\(_{1}\)COCH\(_{2}\)Br (20 mmol) and 13 (22 mmol) in EtOAc (40 mL) was heated to reflux over night. The precipitate was filtrated to afford 14 as a white solid, which was used for the next reaction without further purification.
The pyridinium salt 14 (20 mmol) was suspended in CH\(_{3}\)CN (100 mL) at 0 \(^{\circ }\)C and then potassium carbonate (40 mmol) was added portionwise to maintain the temperature at 0 \(^{\circ }\)C. After 30 min, ethyl propiolate (22 mmol) was added dropwise at 0 \(^{\circ }\)C and the resulting mixture was stirred at room temperature for 3 days. The solvent was evaporated under reduced pressure and the residue was diluted with water and extracted three times with CH\(_{2}\)Cl\(_{2}\). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, and evaporated. The crude product was purified by silica gel chromatography to afford compound 15. LiOH\(\cdot \)H\(_{2}\)O (2.0 mmol) was added to a solution of compound 15 (1.0 mmol) in THF (15 mL), EtOH (15 mL), and H\(_{2}\)O (5 mL). The reaction mixture was stirred at room temperature overnight. The solvent was evaporated under reduced pressure and 1N HCl solution was added to the residue. The precipitated product was filtrated and washed with water to afford 16 which was used for the next reaction without further purification.
The crude carboxylic acid 16 (0.25 mmol) was dissolved in THF (10 mL), then EDC (0.375 mmol), HOBt (0.375 mmol), DIPEA (0.5 mmol), and aralkylamine (0.275 mmol) were added. The reaction mixture was stirred overnight at room temperature. After the solvent was removed, the residue was extracted with CH\(_{2}\)Cl\(_{2}\). The combined organic extracts were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was concentrated and the residue was purified by silica gel chromatography to afford 3/4.
Ethyl 3-benzoyl-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (3a)
Yellow solid; Yield: 52.7 %; Mp: 174–177 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.97 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.86 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.2 Hz, 1H, ArH), 8.00–7.90 (m, 1H, NH), 7.87 (s, 1H, indolizine-H-8), 7.83 (d, \(J\) = 7.3 Hz, 2H, ArH), 7.72 (t, \(J\) = 7.4 Hz, 1H, ArH), 7.64-7.57 (m, 2H, ArH), 7.53 (t, \(J\) = 7.3 Hz, 2H, ArH), 7.36 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6), 7.29–7.18 (m, 1H, ArH), 4.82 (d, \(J\) = 4.3 Hz, 2H, Ar-CH\(_{2})\), 4.41 (d, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.1, 164.0, 155.8, 149.3, 139.7, 138.7, 137.1, 132.8, 132.0, 129.4, 129.2, 128.7, 123.5, 122.8, 122.4, 118.2, 113.7, 108.6, 60.6, 45.0, 14.7; ESI-MS m/z: 428.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{4}\): C, 70.00; H, 4.93; N, 977; Found: C, 70.25; H, 4.95; N, 9.83.
Ethyl 3-benzoyl-7-(pyridin-3-ylmethylcarbamoyl) indolizine-1-carboxylate (3b)
Yellow solid; Yield: 58.5 %; Mp: 209–212 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.88 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.75 (s, 1H, indolizine-H-2), 8.60 (s, 1H, ArH), 8.52 (d, \(J\) = 4.0 Hz, 1H, ArH), 7.81 (s, 1H, indolizine-H-8), 7.80-7.77 (m, 2H, ArH + NH), 7.74 (d, \(J\) = 7.9 Hz, 1H, ArH), 7.60 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.55–7.48 (m, 3H, ArH + indolizine-H-6), 7.42 (t, \(J\) = 5.5 Hz, 1H, ArH), 7.29–s7.25 (m, 1H, ArH), 4.69 (d, \(J\) = 5.8 Hz, 2H, Ar- CH\(_{2})\), 4.32 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.35 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.5, 163.9, 149.6, 149.3, 139.5, 138.6, 136.1, 133.8, 132.4, 132.1, 129.2, 129.1, 129.1, 128.7, 123.9, 123.5, 117.9, 113.7, 108.4, 60.6, 41.9, 14.6; ESI-MS m/z: 428.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{4}\): C, 70.00; H, 4.93; N, 977; Found: C, 70.25; H, 4.95; N, 9.83.
Ethyl 3-benzoyl-7-(furan-2-ylmethylcarbamoyl) indolizine-1-carboxylate (3c)
White solid; Yield: 60.1 %; Mp: 172-174 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.90 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.73 (s, 1H, indolizine-H-2), 7.82 (s, 1H, indolizine-H-8), 7.82–7.78 (m, 2H, ArH), 7.60 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.57–7.46 (m, 3H, ArH), 7.39 (s, 1H, NH), 6.88 (t, \(J\) = 4.4 Hz, 1H, ArH), 6.41–6.30 (m, 2H, ArH), 4.69 (d, \(J\) = 5.3 Hz, 2H, Ar-CH\(_{2})\), 4.36 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.38 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.0, 164.0, 150.8, 142.7, 139.6, 138.6, 132.6, 132.0, 129.2, 129.2, 128.7, 123.5, 117.8, 113.7, 110.8, 108.4, 108.3, 60.6, 37.4, 14.6; ESI-MS m/z: 417.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{24}\)H\(_{20}\)N\(_{2}\)O\(_{5}\): C, 69.19; H, 4.86; N, 6.64; Found: C, 69.22; H, 4.84; N, 6.73.
Ethyl 3-benzoyl-7-(benzylcarbamoyl)indolizine-1- carboxylate (3d)
White solid; Yield: 35.2 %; Mp: 196–198 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.90 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.73 (s, 1H, indolizine-H-2), 7.82 (s, 1H, indolizine-H-8), 7.82–7.78 (m, 2H, ArH), 7.60 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.56–7.46 (m, 3H, ArH), 7.39 (s, 1H, NH), 6.89 (t, \(J\) = 4.4 Hz, 1H, ArH), 6.41-6.29 (m, 2H, ArH), 4.69 (d, \(J\) = 5.3 Hz, 2H, Ar-CH\(_{2})\), 4.36 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.38 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.0, 164.0, 150.8, 142.7, 139.6, 138.6, 132.6, 132.0, 129.2, 129.2, 128.7, 123.5, 117.8, 113.7, 110.8, 108.4, 108.3, 60.6, 37.4, 14.6; ESI-MS m/z: 427.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{22}\)N\(_{2}\)O\(_{4}\): C, 73.29; H, 5.18; N, 6.47; Found: C, 73.23; H, 5.20; N, 6.57.
Ethyl 3-benzoyl-7-(3-fluorobenzylcarbamoyl) indolizine-1-carboxylate (3e)
White solid; Yield: 32.2 %; Mp: 208–210 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.93 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.75 (s, 1H, indolizine-H-2), 7.87–7.78 (m, 3H, ArH + indolizine-H-8), 7.61 (t, \(J\) = 7.1 Hz, 1H, ArH), 7.58–7.49 (m, 3H, ArH), 7.38–7.28 (m, 1H, ArH), 7.16 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6), 7.09 (d, \(J\) = 9.4 Hz, 1H, ArH), 7.00 (t, \(J\) = 8.3 Hz, 1H, ArH), 6.97–6.88 (m, 1H, NH), 4.68 (d, \(J\) = 5.4 Hz, 2H, Ar-CH\(_{2})\), 4.36 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.38 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.2, 164.1, 140.5, 139.6, 138.7, 132.5, 132.1, 130.6, 130.6, 129.3, 129.1, 128.7, 123.7, 123.5, 117.7, 115.2, 115.0, 114.9, 114.8, 113.8, 108.4, 60.7, 44.0, 14.7; ESI-MS m/z: 445.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{21}\)FN\(_{2}\)O\(_{4}\): C, 70.26; H, 4.76; N, 6.30; Found: C, 70.36; H, 4.97; N, 6.08.
(R)-Ethyl 3-benzoyl-7-(1-phenylethylcarbamoyl) indolizine-1-carboxylate (3f)
White solid; Yield: 79.1 %; Mp: 175–178 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.92 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.71 (s, 1H, indolizine-H-2), 7.87–7.78 (m, 3H, ArH + indolizine-H-8), 7.60 (t, \(J\) = 7.0 Hz, 1H, ArH), 7.53 (t, \(J\) = 7.2 Hz, 3H, ArH), 7.46–7.41 (m, 2H, ArH), 7.38 (t, \(J\) = 7.3 Hz, 2H, ArH), 7.31 (d, \(J\) = 7.2 Hz, 1H, ArH), 6.66 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 5.43–5.30 (m, 1H, Ar-CH), 4.37 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.66 (s, 3H, CH\(_{3})\), 1.39 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 164.3, 164.1, 142.9, 139.6, 138.7, 133.0, 132.0, 129.2, 129.2, 129.1, 128.7, 127.9, 126.5, 123.5, 117.5, 113.9, 108.2, 60.6, 49.9, 21.9, 14.7; ESI-MS m/z: 4451.0 [M+H]\(^{+}\), 881.3 [2M+H]\(^{+}\); Anal. Calcd. for C\(_{27}\)H\(_{24}\)N\(_{2}\)O\(_{4}\): C, 73.37; H, 5.39; N, 6.32; Found: C, 73.62; H, 5.49; N, 6.36.
(S)-Ethyl 3-benzoyl-7-(2-methoxy-2-oxo-1- phenylethylcarbamoyl)indolizine-1-carboxylate (3g)
White solid; Yield: 72.4 %; Mp: 183–185 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.92 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.80 (s, 1H, indolizine-H-2), 7.87–7.83 (m, 2H, ArH), 7.82 (s, 1H, indolizine-H-8), 7.60 (t, \(J\) = 6.9 Hz, 1H, NH), 7.56–7.45 (m, 6H, ArH), 7.44-7.36 (m, 3H, ArH), 5.81 (d, \(J\) = 6.8 Hz, 1H, Ar-CH), 4.38 (d, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 3.80 (s, 3H, CO\(_{2}\)CH\(_{3})\), 1.40 (t, \(J\) = 6.8 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 171.4, 164.5, 163.9, 139.6, 138.5, 136.3, 132.1, 131.9, 129.4, 129.3, 129.2, 129.0, 128.7, 127.7, 123.6, 118.3, 113.6, 108.7, 60.7, 57.3, 53.2, 14.7; ESI-MS m/z: 485.2 [M+H]\(^{+}\); Anal. Calcd. for C\(_{28}\)H\(_{24}\)N\(_{2}\)O\(_{6}\): C, 69.47; H, 5.02; N, 5.94; Found: C, 69.41; H, 4.99; N, 5.78.
(R)-Ethyl 3-benzoyl-7-(2-methoxy-2-oxo-1- phenylethylcarbamoyl)indolizine-1-carboxylate (3h)
Brown solid; Yield: 100 %; Mp: 188–190 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.92 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.80 (s, 1H, indolizine-H-2), 7.87–7.82 (m, 2H, ArH), 7.82 (s, 1H, indolizine-H-8), 7.60 (t, \(J\) = 6.9 Hz, 1H, NH), 7.57–7.44 (m, 6H, ArH), 7.44–7.36 (m, 3H, ArH + indolizine-H-6), 5.81 (d, \(J\) = 6.8 Hz, 1H, Ar-CH), 4.38 (q, \(J\) = 6.8 Hz, 2H, CH\(_{2})\), 3.80 (s, 3H, CO\(_{2}\)CH\(_{3})\), 1.40 (t, \(J\) = 6.8 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 171.4, 164.5, 163.9, 139.6, 138.5, 136.3, 132.1, 131.9, 129.4, 129.3, 129.2, 129.0, 128.7, 127.7, 123.6, 118.3, 113.6, 108.7, 60.7, 57.3, 53.2, 14.7; ESI-MS m/z: 485.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{28}\)H\(_{24}\)N\(_{2}\)O\(_{6}\): C, 69.47; H, 5.02; N, 5.94; Found: C, 69.41; H, 4.99; N, 5.78.
Ethyl 7-(benzo[d][1,3]dioxol-5-ylmethylcarbamoyl)-3-benzoylindolizine-1-carboxylate (3i)
Off white solid; Yield: 99.3 %; Mp: 181–183 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.91 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.72 (s, 1H, indolizine-H-2), 7.83 (s, 1H, indolizine-H-8), 7.82–7.79 (m, 2H, ArH), 7.61 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.53 (t, \(J\) = 7.4 Hz, 3H, ArH), 6.89–6.80 (m, 3H, ArH+ indolizine-H-6), 6.77 (d, \(J\) = 7.9 Hz, 1H, ArH), 5.95 (s, 2H, ArO-CH\(_{2})\), 4.58 (d, \(J\) = 5.3 Hz, 2H, Ar-CH\(_{2})\), 4.35 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.38 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.1, 164.1, 148.3, 147.5, 139.6, 138.7, 132.8, 132.0, 131.7, 129.2, 129.1, 128.7, 123.5, 121.6, 117.6, 113.8, 108.8, 108.6, 108.3, 101.4, 60.6, 44.5, 14.7; ESI-MS m/z: 470.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{27}\)H\(_{22}\)N\(_{2}\)O\(_{6}\): C, 68.75; H, 4.68; N, 5.94; Found: C, 68.93; H, 4.71; N, 5.95.
Ethyl 3-benzoyl-7-(naphthalen-2-ylmethylcarbamoyl)indolizine-1-carboxylate (3j)
White solid; Yield: 84.0 %; Mp: 156–158 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.88 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.74 (s, 1H, indolizine-H-2), 7.89–7.73 (m, 7H, ArH + indolizine-H-8), 7.65–7.56 (m, 1H, ArH), 7.56-741 (m, 6H, ArH + indolizine-H-6), 7.09–6.98 (m, 1H, NH), 4.83 (d, \(J\) = 5.1 Hz, 2H, Ar-CH\(_{2})\), 4.32 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.35 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.2, 164.0, 139.6, 138.7, 135.3, 133.6, 133.1, 132.8, 132.0, 129.2, 129.2, 128.9, 128.7, 127.9, 127.9, 127.0, 126.6, 126.3, 126.2, 123.5, 117.8, 113.8, 108.3, 60.6, 44.8, 14.7; ESI-MS m/z: 477.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{30}\)H\(_{24}\)N\(_{2}\)O\(_{4}\): C, 75.61; H, 5.08; N, 5.88; Found: C, 75.53; H, 5.19; N, 5.01.
Ethyl 3-benzoyl-7-(2,3-dihydro-1H-inden-2- ylcarbamoyl)indolizine-1-carboxylate (3k)
White solid; Yield: 66.4 %; Mp: 217–219 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.91 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.66 (s, 1H, indolizine-H-2), 7.83 (s, 1H, indolizine-H-8), 7.82–7.79 (m, 2H, ArH), 7.60 (t, \(J\) = 7.2 Hz, 1H, ArH), 7.56–7.49 (m, 3H, ArH), 7.31–7.24 (m, 1H, ArH), 7.24–7.16 (m, 2H, 2H), 6.73 (d, \(J\) = 7.2 Hz, 1H, ArH), 5.05–4.90 (m, 1H, CH), 4.35 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 3.48 (d, \(J\) = 7.2 Hz, 1H, Ar-CH\(_{2})\), 3.44 (d, \(J\) = 7.2 Hz, 1H, Ar-CH\(_{2})\), 3.02 (d, \(J\) = 3.8 Hz, 1H, Ar-CH\(_{2})\), 2.98 (d, \(J\) = 4.1 Hz, 1H, Ar-CH\(_{2})\), 1.36 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 186.0, 165.1, 164.1, 140.9, 139.6, 138.7, 133.0, 132.1, 129.2, 128.7, 127.2, 125.1, 123.5, 117.6, 113.9, 108.3, 60.6, 51.7, 40.3, 14.7; ESI-MS m/z: 453.0 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{28}\)H\(_{24}\)N\(_{2}\)O\(_{4 }\)[M+H]\(^{+}\):453.1814; Found 453.1813.
Ethyl 3-benzoyl-7-(phenethylcarbamoyl) indolizine-1-carboxylate (3l)
White solid; Yield: 34.8 %; Mp: 198–200 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \quad \delta \) 9.89 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.65 (s, 1H, indolizine-H-2), 7.8 (s, 1H, indolizine-H-8), 7.72 (d, \(J\) = 7.2 Hz, 2H, ArH), 7.48 (d, \(J\) = 7.3 Hz, 1H, ArH) 7.44 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.34–7.32 (m, 2H, ArH), 7.30–7.25 (m, 3H, ArH), 6.46–6.35 (m, 1H, NH), 4.37 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 3.76 (q, \(J\) = 6.3 Hz, 2H, ArCH\(_{2}\)-CH\(_{2})\), 2.98 (t, \(J\) = 6.8 Hz, 2H, Ar-CH\(_{2})\), 1.38 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 165.3, 164.0, 139.6, 138.8, 138.7, 133.1, 132.0, 129.2, 129.2, 129.0, 128.7, 126.9, 123.4, 117.6, 113.6, 108.3, 60.6, 41.7, 35.9, 14.7; ESI-MS m/z: 441.1 [M+H]\(^{+}\), 881.2 [2M+H]\(^{+}\).
(S)-Ethyl 3-benzoyl-7-(1-methoxy-1-oxo-3-phenylpropan-2-ylcarbamoyl)indolizine-1-carboxylate (3m)
Yellowish green solid; Yield: 34.8 %; Mp: 113–115 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.88 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.70 (s, 1H, indolizine-H-2), 7.84 (s, 1H, indolizine-H-8), 7.84–7.81 (m, 2H, ArH), 7.61 (t, \(J\) = 7.2 Hz, 1H, ArH), 7.54 (t, \(J\) = 7.3 Hz, 2H, ArH), 7.39 (d, \(J\) = 7.2 Hz, 1H, ArH), 7.37–7.31 (m, 2H, ArH), 7.29 (d, \(J\) = 5.6 Hz, 1H, ArH), 7.22 (d, \(J\) = 7.3 Hz, 2H, ArH), 7.06 (d, \(J\) = 7.5 Hz, 1H, NH), 5.14 (q, \(J\) = 6.3 Hz, 1H, ArCH\(_{2}\)-CH), 4.38 (q, \(J\) = 7.1 Hz, 2H, CH\(_{2})\), 3.81 (s, 3H, CO\(_{2}\)CH\(_{3})\), 3.41–3.22 (m, 2H, Ar-CH\(_{2})\), 1.39 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 185.9, 172.0, 164.8, 163.7, 139.5, 138.4, 135.9, 132.1, 132.0, 129.9, 129.4, 129.2, 129.1, 128.9, 128.6, 127.5, 123.5, 118.2, 113.3, 108.6, 60.6, 54.0, 52.7, 38.1, 14.6; ESI-MS m/z: 499.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{29}\)H\(_{26}\)N\(_{2}\)O\(_{6}\): C, 69.87; H, 5.26; N, 5.62; Found: C, 68.90; H, 5.14; N, 5.46.
(S)-Ethyl 7-(3-(1H-imidazol-5-yl)-1-methoxy-1-oxopropan-2-ylcarbamoyl)-3-benzoylindolizine-1-carboxy late (3n)
Yellow solid; Yield: 57.4 %; Mp: 161–163 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.91 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 9.00 (d, \(J\) = 5.7 Hz, 1H, NH), 8.95 (s, 1H, indolizine-H-2), 7.82 (s, 2H, ArH), 7.80 (s, 1H, indolizine-H-8), 7.72 (s, 1H, ArH), 7.63–7.57 (m, 1H, ArH), 7.57–7.48 (m, 3H, ArH), 6.88 (s, 1H, ArH), 4.99 (q, \(J\) = 5.3 Hz, 1H, ArCH\(_{2}\)-CH), 4.38 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 3.71 (s, 3H, CO\(_{2}\)CH\(_{3})\), 3.25 (qd, \(J\) = 14.9, 4.7 Hz, 2H, Ar-CH\(_{2})\), 1.39 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 171.9, 165.0, 163.9, 139.7, 138.8, 135.7, 132.3, 132.0, 129.4, 129.2, 129.0, 128.7, 123.4, 118.8, 114.9, 113.5, 108.7, 60.6, 53.5, 52.6, 29.0, 14.7; ESI-MS m/z: 489.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{24}\)N\(_{4}\)O\(_{6}\): C, 63.93; H, 4.95; N, 11.47; Found: C, 63.77; H, 5.140; N, 11.65.
(S)-Ethyl 7-(3-(1H-indol-3-yl)-1-methoxy-1-oxopropan-2-ylcarbamoyl)-3-benzoylindolizine-1-carboxylate (3o)
Light yellow solid; Yield: 74.6 %; Mp: 191–193 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.85 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.71 (s, 1H, indolizine-H-2), 8.34 (br, 1H, NH), 7.86–7.81 (m, 2H, ArH), 7.81 (s, 1H, indolizine-H-8), 7.64–7.49 (m, 4H, ArH), 7.38–7.33 (m, 2H, ArH), 7.16 (d, \(J\) = 7.4 Hz, 1H, ArH), 7.13–7.07 (m, 2H, ArH), 6.94 (m, 1H, ArH), 5.16 (q, \(J\) = 7.0 Hz, 1H, ArCH\(_{2}\)-CH), 4.36 (d, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 3.75 (s, 3H, CO\(_{2}\)CH\(_{3})\), 3.49 (t, \(J\) = 4.3 Hz, 2H, Ar-CH\(_{2})\), 1.36 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.0, 172.3, 164.9, 163.9, 139.6, 138.5, 136.4, 132.3, 132.1, 129.3, 129.2, 129.1, 128.7, 127.7, 123.5, 123.2, 122.6, 120.1, 118.7, 118.5, 113.3, 111.6, 110.1, 108.6, 60.6, 53.7, 52.8, 27.9, 14.6; ESI-MS m/z: 538.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{31}\)H\(_{27}\)N\(_{3}\)O\(_{6}\): C, 69.02; H, 4.99; N, 7.62; Found: C, 69.26; H, 5.06; N, 7.82.
Ethyl 3-(2-fluorobenzoyl)-7-(pyridin-2- ylmethylcarbamoyl)indolizine-1-carboxylate (4a)
Yellow solid; Yield: 97.0 %; Mp: 178–181 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.03 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.86 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 4.3 Hz, 1H, ArH), 8.03–7.95 (br, 1H, NH), 7.76–7.69 (m, 2H, ArH + indolizine-H-8), 7.63 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.61–7.49 (m, 2H, ArH), 7.37 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.33–7.18 (m, 4H, ArH), 4.82 (d, \(J\) = 4.0 Hz, 2H, Ar-CH\(_{2})\), 4.39 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.42 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 182.0, 165.0, 163.8, 159.7 (d, \(^{1}J_\mathrm{C-F}\) = 251.3 Hz), 155.7, 149.2, 139.0, 137.3, 133.2, 132.7 (d, \(^{3}J_\mathrm{C-F}\) = 8.4 Hz), 132.7, 130.3, 129.8, 129.9, 128.2 (d, \(^{2}J_\mathrm{C-F}\) = 15.8 Hz), 124.5, 124.0, 122.9, 122.5, 118.3, 116.7 (d, \(^{2}J_\mathrm{C-F}\) = 21.6 Hz), 114.1, 109.1, 60.7, 45.0, 14.7; ESI-MS m/z: 446.2 [M+H]\(^{+}\).
Ethyl 3-(2-chlorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4b)
White solid; Yield:52.0 %; Mp: 169–171 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.06 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.87 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 4.6 Hz, 1H, ArH), 8.00 (br, 1H, NH), 7.73 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.64 (d, \(J\) = 7.2 Hz, 1H, ArH), 7.56 (s, 1H, indolizine-H-8), 7.55–7.43 (m, 4H, ArH), 7.43–7.34 (m, 2H, ArH), 7.26 (d, \(J\) = 6.9 Hz, 1H, ArH), 4.82 (d, \(J\) = 4.0 Hz, 2H, Ar-CH\(_{2})\), 4.39 (q, \(J\) = 7.4 Hz, 2H, CH\(_{2})\), 1.42 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.2, 165.0, 163.8, 155.7, 149.2, 139.1, 139.0, 137.3, 133.4, 131.6, 131.3, 130.5, 130.0, 129.4, 129.3, 126.9, 123.6, 122.9, 122.5, 118.3, 114.2, 109.2, 100.2, 60.7, 45.0, 14.7; ESI-MS m/z: 462.0 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{25}\)H\(_{20}\)ClN\(_{3}\)O\(_{4 }\)[M+H]\(^{+}\):462.1221; Found 462.1210.
Ethyl 3-(2-bromobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4c)
Yellow solid; Yield: 87.1 %; Mp: 156–158 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.08 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.89 (s, 1H, indolizine-H-2), 8.62 (d, \(J\) = 3.4 Hz, 1H, ArH), 8.04 (br, 1H, NH), 7.79 (t, \(J\) = 7.2 Hz, 1H, ArH), 7.71 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.67 (d, \(J\) = 6.1 Hz, 1H, ArH), 7.56 (s, 1H, indolizine-H-8), 7.50–7.36 (m, 4H, ArH), 7.35–7.29 (m, 1H, ArH), 4.85 (d, \(J\) = 4.3 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 185.0, 165.0, 163.8, 155.7, 149.3, 141.0, 139.2, 137.2, 133.6, 133.4, 131.4, 130.1, 129.4, 129.2, 127.4, 122.8, 122.4, 120.1, 118.3, 114.2, 109.2, 60.7, 45.0, 14.7; ESI-MS m/z: 505.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)BrN\(_{3}\)O\(_{4}\): C, 59.20; H, 4.00; N, 8.19; Found: C, 59.30; H, 3.98; N, 8.30.
Ethyl 3-(2-trifluorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4d)
White solid; Yield: 48.5 %; Mp: 143–145 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.04 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.88 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.5 Hz, 1H, ArH), 8.07 (br, 1H, NH), 7.83 (d, \(J\) = 6.8 Hz, 1H, ArH), 7.76 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.72–7.61 (m, 3H, ArH), 7.56 (d, \(J\) = 7.4 Hz, 1H, ArH), 7.50 (s, 1H, indolizine-H-8), 7.40 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.29 (t, \(J\) = 6.0 Hz, 1H, ArH), 4.84 (d, \(J\) = 4.4 Hz, 2H), 4.39 (q, \(J\) = 7.1 Hz, 2H), 1.42 (t, \(J\) = 7.1 Hz, 3H); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.6, 165.0, 163.7, 155.5, 148.9, 139.1, 138.1, 137.5, 133.4, 131.7, 130.2, 129.9, 129.4, 128.9, 128.2, 127.1, 123.5, 122.9, 122.6, 118.4, 114.2, 109.1, 60.7, 44.8, 14.6; ESI-MS m/z: 495.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{20}\)F\(_{3}\)N\(_{3}\)O\(_{4}\): C, 63.01; H, 4.16; N, 8.42; Found: C, 63.03; H, 4.07; N, 8.48.
Ethyl 3-(2-nitrobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4e)
Yellow solid; Yield: 76.0 %; Mp: 162–164 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.98 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.85 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 4.1 Hz, 1H, ArH), 8.25 (d, \(J\) = 8.2 Hz, 1H, ArH), 8.02 (br, 1H, NH), 7.80 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.72 (q, \(J\) = 6.7 Hz, 2H, ArH), 7.65 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.60 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-6), 7.44 (s, 1H, indolizine-H-8), 7.36 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.29–7.23 (m, 1H, ArH), 4.82 (d, \(J\) = 4.1 Hz, 2H, Ar-CH\(_{2})\), 4.37 (q, \(J\) = 7.1 Hz, 2H, CH\(_{2})\), 1.40 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 182.5, 164.9, 163.6, 155.7, 149.2, 146.9, 139.1, 137.2, 135.9, 134.1, 133.4, 130.9, 129.5, 129.3, 128.4, 125.1, 123.1, 122.8, 122.4, 118.4, 114.2, 109.2, 60.7, 45.0, 14.6; ESI-MS m/z: 473.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)N\(_{4}\)O\(_{6}\): C, 63.38; H, 4.31; N, 11.75; Found: C, 63.56; H, 4.27; N, 11.86.
Ethyl 3-(2-aminoobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4f)
Brown solid; Yield:68.2 %; Mp: 73–75 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.68 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.81 (s, 1H, indolizine-H-2), 8.62–8.55 (m, 1H, ArH), 8.01–8.00 (br, 1H, NH), 7.75 (s, 1H, indolizine-H-8), 7.70 (t, \(J\) = 7.5 Hz, 1H, ArH), 7.62 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.51 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-6), 7.35 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.32–7.26 (m, 1H, ArH), 7.26–7.20 (m, 1H, ArH), 6.78-6.69 (m, 2H, ArH), 5.76–5.30 (br, 2H, NH\(_{2})\), 4.80 (d, \(J\) = 3.9 Hz, 2H, Ar-CH\(_{2})\), 4.38 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2})\), 1.42 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.9, 165.3, 164.1, 155.9, 149.4, 149.2, 138.3, 137.2, 133.4, 132.4, 132.1, 128.8, 128.6, 124.2, 122.8, 122.4, 120.9, 118.1, 117.1, 116.6, 113.2, 108.1, 60.5, 45.0, 14.7; ESI-MS m/z: 443.0 [M+H]\(^{+}\).
Ethyl 3-(3-fluorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4g)
Off white solid; Yield: 73.3 %; Mp: 169–171 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \quad \delta \) 9.94 (d, \(J\) = 6.5 Hz, 1H, indolizine-H-5), 8.86 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.0 Hz,1H, ArH), 8.04–7.94 (br, 1H, NH), 7.86 (s, 1H, indolizine-H-8), 7.72 (t, \(J\) = 7.5 Hz, 1H, ArH), 7.61 (d, \(J\) = 6.3 Hz, 2H, ArH+ indolizine-H-6), 7.53 (d, \(J\) = 8.3 Hz, 2H, ArH), 7.36 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.34–7.23 (m, 2H, ArH), 4.81 (d, \(J\) = 3.9 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 6.8 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \quad \delta \) 184.2, 165.1, 164.2 (d, \(^{1}J_{C-F}\) = 274.0 Hz), 162.8, 155.7, 149.3, 141.7, 138.9, 137.1, 133.1, 130.4 (d, \(^{3}J_{C-F}\) = 7.7 Hz), 129.4, 129.2, 125.0 (d, \(^{3}J_{C-F}\) = 3.0 Hz), 123.1, 122.8, 122.4, 119.0 (d, \(^{2}J_{C-F}\) = 21.3 Hz), 118.2, 116.1 (d, \(^{2}J_{C-F}\) = 22.7 Hz), 113.9, 108.8, 60.7, 45.0, 14.7; ESI-MS m/z: 446.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)FN\(_{3}\)O\(_{4}\): C, 67.24; H, 4.52; N, 9.36; Found: C, 67.41; H, 4.53; N, 9.43.
Ethyl 3-(3-chlorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4h)
Light yellow solid; Yield: 86.2 %; Mp: 157–160 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.94 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.87 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.1 Hz, 1H, ArH), 8.00 (br, 1H, NH), 7.84 (s, 1H, indolizine-H-8), 7.80 (s, 1H, ArH), 7.76–7.67 (m, 2H, ArH), 7.61 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-6), 7.57 (d, \(J\) = 7.9 Hz, 1H, ArH), 7.47 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.36 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.29–7.23 (m, 1H, ArH), 4.81 (d, \(J\) = 4.1 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 7.1 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.2, 165.0, 163.9, 155.7, 149.3, 141.3, 138.9, 137.2, 134.9, 133.2, 131.9, 130.0, 129.4, 129.2, 129.1, 127.3, 123.1, 122.8, 122.5, 118.2, 114.0, 108.8, 60.7, 45.0, 14.7; ESI-MS m/z: 461.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)ClN\(_{3}\)O\(_{4}\): C, 65.01; H, 4.36; N, 9.10; Found: C, 65.14; H, 4.44; N, 9.08.
Ethyl 3-(3-bromobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4i)
Yellow solid; Yield: 57.3 %; Mp: 124-127 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.90 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.85 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 4.6 Hz, 1H, ArH), 8.07 (t, \(J\) = 4.2 Hz, 1H, NH), 7.94 (s, 1H, indolizine-H-8), 7.82 (s, 1H, ArH), 7.72 (t, \(J\) = 5.8 Hz, 3H, ArH), 7.59 (dd, \(J\) = 7.3, 1.8 Hz, 1H, indolizine-H-6), 7.40 (t, \(J\) = 7.8 Hz, 1H, ArH), 7.36 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.27–7.22 (m, 1H, ArH), 4.81 (d, \(J\) = 4.6 Hz, 2H, Ar-CH\(_{2})\), 4.40 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.43 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.0, 165.0, 163.7, 155.8, 149.2, 141.5, 138.9, 137.1, 134.8, 133.1, 131.9, 130.2, 129.3, 129.1, 127.7, 123.0, 122.9, 122.8, 122.4, 118.2, 113.9, 108.8, 60.7, 45.0, 14.6; ESI-MS m/z: 505.9, 507.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)BrN\(_{3}\)O\(_{4}\): C, 59.23; H, 3.97; N, 8.35; Found: C, 59.30; H, 3.98; N, 8.30.
Ethyl 3-(3-trifluorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4j)
Light yellow solid; Yield: 85.9 %; Mp: 184–186 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.97 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.88 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.3 Hz, 1H, ArH), 8.09 (br, 1H, NH), 8.01 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.97 (s, 1H, indolizine-H-8), 7.86 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.81 (s, 1H, ArH), 7.73 (t, \(J\) = 7.9 Hz, 1H, ArH), 7.68 (t, \(J\) = 7.9 Hz, 1H, ArH), 7.63 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.36 (d, \(J\) = 7.9 Hz, 1H, ArH), 7.27–7.24 (m, 1H, ArH), 4.82 (d, \(J\) = 4.0 Hz, 2H, Ar-CH2), 4.42 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 184.2, 165.0, 163.8, 155.7, 149.3, 140.4, 139.1, 137.2, 133.3, 132.3, 129.4, 129.3, 128.5 (q, \(^{3}J_{C-F }\)= 3.6 Hz), 126.0 (q, \(^{3}J_{C-F }\)= 3.9 Hz), 123.0, 122.8, 122.5, 118.2, 114.1, 109.0, 60.7, 45.0, 14.6; ESI-MS m/z: 496.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{20}\)F\(_{3}\)N\(_{3}\)O\(_{4}\): C, 63.94; H, 4.17; N, 8.50; Found: C, 63.03; H, 4.07; N, 8.48.
Ethyl 3-(3-nitrobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4k)
Light yellow solid; Yield: 63.6 %; Mp: 172–174 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.95 (d, \(J\) = 6.7 Hz, 1H, indolizine-H-5), 8.88 (s, 1H, indolizine-H-2), 8.66 (s, 1H, ArH), 8.60 (d, \(J\) = 4.4 Hz, 1H, ArH), 8.45 (d, \(J\) = 8.2 Hz, 1H, ArH), 8.15 (d, \(J\) = 7.5 Hz, 1H, ArH), 8.08 (s, 1H, NH), 7.80 (s, 1H, indolizine-H-8), 7.74 (t, \(J\) = 8.0 Hz, 2H, ArH), 7.65 (d, \(J\) = 7.0 Hz, 1H, indolizine-H-6), 7.38 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.30–7.26 (m, 1H, ArH), 4.82 (d, \(J\) = 4.3 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 6.7 Hz, 2H, CH\(_{2})\), 1.43 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 182.9, 164.9, 163.6, 155.7, 149.2, 148.4, 141.2, 139.2, 137.3, 134.7, 133.6, 129.9, 129.4, 129.3, 126.3, 124.0, 122.9, 122.6, 122.5, 118.3, 114.3, 109.3, 60.8, 45.0, 14.7; ESI-MS m/z: 473.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)N\(_{4}\)O\(_{6}\): C, 63.36; H, 4.33; N, 11.75; Found: C, 63.56; H, 4.27; N, 11.86.
Ethyl 3-(3-aminoobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4l)
Light yellow solid; Yield: 80.3 %; Mp: 168-171 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.88 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.82 (s, 1H, indolizine-H-2), 8.58 (d, \(J\) = 3.7 Hz, 1H, ArH), 8.08 (s, 1H, NH), 7.87 (s, 1H, indolizine-H-8), 7.71 (t, \(J\) = 7.6 Hz, 1H, ArH), 7.54 (d, \(J\) = 7.3 Hz, 1H, NH), 7.36 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.32–7.20 (m, 2H, ArH), 7.16 (d, \(J\) = 7.5 Hz, 1H, ArH), 7.11 (s, 1H, ArH), 6.88 (d, \(J\) = 7.8 Hz, 1H, ArH), 4.80 (d, \(J\) = 3.7 Hz, 2H, Ar-CH\(_{2})\), 4.38 (q, \(J\) = 7.0 Hz, 2H), 3.68 (s, 2H, NH\(_{2})\), 1.41 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 186.1, 165.2, 164.0, 156.0, 149.2, 146.9, 140.7, 138.5, 137.1, 132.6, 129.5, 129.3, 129.1, 123.5, 122.7, 122.4, 119.5, 118.5, 118.1, 115.2, 113.5, 108.3, 60.6, 45.1, 14.7; ESI-MS m/z: 443.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{22}\)N\(_{4}\)O\(_{4}\): C, 67.82; H, 4.91; N, 12.57; Found: C, 67.86; H, 5.01; N, 12.66.
Ethyl 3-(4-fluorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4m)
White solid; Yield: 67.4 %; Mp: 179–181 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.88 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.84 (s, 1H, indolizine-H-2), 8.58 (d, \(J\) = 3.5 Hz, 1H, ArH), 8.11 (s, 1H, NH), 7.90–7.82 (m, 2H, ArH), 7.81 (s, 1H, indolizine-H-8), 7.72 (t, \(J\) = 7.6 Hz, 1H, ArH), 7.57 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.36 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.27–7.15 (m, 3H, ArH), 4.81 (d, \(J\) = 3.3 Hz, 2H, Ar-CH\(_{2})\), 4.39 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.42 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.4, 165.1 (d, \(^{1}J_{C-F}\) = 251.0 Hz), 165.0, 163.8, 155.9, 149.2, 138.6, 137.1, 135.8 (d, \(^{3}J_{C-F}\) = 2.9 Hz), 132.8, 131.7, 131.6, 129.0, 128.7, 123.2, 122.7, 122.4, 118.2, 115.9, 115.7 (d, \(^{2}J_{C-F}\) = 22.0 Hz), 113.7, 108.6, 60.6, 45.0, 14.6; ESI-MS m/z: 446.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)FN\(_{3}\)O\(_{4}\): C, 67.41; H, 4.53; N, 9.43; Found: C, 67.27; H, 4.61; N, 9.27.
Ethyl 3-(4-chlorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4n)
Yellow solid; Yield: 97.6 %; Mp: 180–182 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.96 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.89 (s, 1H, indolizine-H-2), 8.62 (d, \(J\) = 4.5 Hz, 1H, ArH), 7.98–7.89 (m, 1H, NH), 7.85 (s, 1H, indolizine-H-8), 7.84–7.78 (m, 2H, ArH), 7.74 (td, \(J\) = 7.7, 1.7 Hz, 1H, ArH), 7.62 (dd, \(J\) = 7.3, 1.9 Hz, 1H, indolizine-H-6), 7.57–7.51 (m, 2H, ArH), 7.37 (d, \(J\) = 7.9 Hz, 1H, ArH), 7.30–7.25 (m, 1H, ArH), 4.83 (d, \(J\) = 4.5 Hz, 2H, Ar-CH\(_{2})\), 4.44 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.46 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.6, 165.1, 163.9, 155.7, 149.3, 138.9, 138.4, 138.1, 137.1, 133.1, 130.6, 129.2, 129.0, 123.2, 122.8, 122.4, 118.2, 113.9, 108.8, 60.7, 45.0, 14.7; ESI-MS m/z: 462.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)ClN\(_{3}\)O\(_{4}\): C, 64.93; H, 4.37; N, 9.20; Found: C, 65.01; H, 4.36; N, 9.10.
Ethyl 3-(4-bromobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4o)
Off white solid; Yield: 91.1 %; Mp: 182–184 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.91 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.85 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 3.9 Hz, 1H, ArH), 8.03 (s, 1H, NH), 7.81 (s, 1H, indolizine-H-8), 7.75–7.63 (m, 5H, ArH), 7.59 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-6), 7.37 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.30–7.22 (m, 1H, ArH), 4.81 (d, \(J\) = 3.7 Hz, 2H, Ar-CH\(_{2})\), 4.40 (q, \(J\) = 6.7 Hz, 2H, CH\(_{2})\), 1.43 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.6, 165.0., 163.8, 155.8, 149.2, 138.8, 138.4, 137.2, 133.0, 132.0, 130.7, 129.2, 129.1, 126.8, 123.1, 122.8, 122.5, 118.2, 113.9, 108.7, 60.7, 45.0, 14.7; ESI-MS m/z: 505.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{20}\)BrN\(_{3}\)O\(_{4}\): C, 59.30; H, 3.98; N, 8.30; Found: C, 59.25; H, 4.10; N, 8.11.
Ethyl 3-(4-trifluorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4p)
Off white solid; Yield: 42.4 %; Mp: 197–199 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.98 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.88 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.2 Hz, 1H, ArH), 8.01 (s, 1H, NH), 7.93 (d, \(J\) = 7.8 Hz, 2H, ArH), 7.84-7.78 (d, \(J\) = 6.9 Hz, 3H, ArH), 7.74 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.63 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.37 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.31–7.23 (m, 1H, ArH), 4.83 (d, \(J\) = 7.7 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 184.5, 165.0, 163.8, 155.6, 149.2, 142.8, 139.1, 137.3, 133.3, 129.5, 129.4, 129.3 (q, \(^{2}J_{C-F}\) = 26 Hz), 125.8 (q, \(^{3}J_{C-F}\) = 3.5 Hz), 123.0, 122.9, 122.5, 118.2, 114.2, 109.0, 60.8, 45.0, 14.7; ESI-MS m/z: 496.1 [M+H]\(^{+}\).
Ethyl 3-(4-nitrobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4q)
Yellow solid; Yield: 76.2 %; Mp: 208–210 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.99 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.89 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.3 Hz, 1H, ArH), 8.40 (d, \(J\) = 8.0 Hz, 2H, ArH), 8.01 (s, 1H, NH), 7.98 (d, \(J\) = 8.2 Hz, 2H, ArH), 7.79 (s, 1H, indolizine-H-8), 7.75 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.67 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.38 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.31–7.24 (m, 1H, ArH), 4.83 (d, \(J\) = 4.1 Hz, 2H, Ar-CH\(_{2})\), 4.42 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 183.5, 164.8, 163.6, 155.5, 149.7, 149.2, 145.1, 139.3, 137.3, 133.7, 130.0, 129.6, 129.3, 124.0, 122.9, 122.8, 122.5, 118.3, 114.4, 109.3, 60.9, 44.9, 14.7; ESI-MS m/z: 473.2 [M+H]\(^{+}\).
Ethyl 3-(4-aminoobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4r)
Yellow solid; Yield: 62.5 %; Mp: 217–219 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.82 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.83 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 4.0 Hz, 1H, ArH), 7.92 (s, 1H, NH), 7.87 (s, 1H, indolizine-H-8), 7.79-7.68 (m, 3H, ArH), 7.51 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.35 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.28–7.21 (m, 1H, ArH), 6.73 (d, \(J\) = 7.7 Hz, 2H, ArH), 4.81 (d, \(J\) = 3.6 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 6.7 Hz, 2H, CH\(_{2})\), 4.20 (br, 2H, NH\(_{2})\), 1.44 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 184.6, 165.4, 164.2, 155.9, 150.8, 149.3, 138.2, 137.1, 132.0, 131.9, 129.9, 129.0, 128.1, 123.9, 122.8, 122.4, 118.2, 114.1, 113.1, 107.9, 60.5, 45.0, 14.7; ESI-MS m/z: 443.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{22}\)N\(_{4}\)O\(_{4}\): C, 67.65; H, 5.06; N, 12.43; Found: C, 67.86; H, 5.01; N, 12.66.
Ethyl 3-(2,4-dihydroxybenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4s)
Yellow solid; Yield: 39.2 %; Mp: 238–240 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, DMSO) \(\delta \) 10.97 (s, 1H, OH), 10.34 (s, 1H, OH), 9.64 (d, \(J\) = 7.1 Hz, 1H, indolizine-H-5), 9.54 (t, \(J\) = 5.8 Hz, 1H, NH), 8.84 (s, 1H, indolizine-H-2), 8.55 (d, \(J\) = 3.9 Hz, 1H, ArH), 7.79 (t, \(J\) = 7.1 Hz, 1H, indolizine-H-6), 7.67 (d, \(J\) = 9.3 Hz, 2H, ArH), 7.53 (d, \(J\) = 8.6 Hz, 1H, ArH), 7.39 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.34–7.25 (m, 1H, ArH), 6.45 (d, \(J\) = 8.6 Hz, 1H, ArH), 6.41 (s, 1H, ArH), 4.64 (d, \(J\) = 5.6 Hz, 2H, Ar-CH\(_{2})\), 4.34 (q, 6.9 Hz, 2H, CH\(_{2})\), 1.35 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, DMSO) \(\delta \) 185.1, 164.3, 162.9, 160.8, 158.3, 148.9, 137.5, 136.7, 132.7, 132.0, 128.2, 127.0, 123.1, 122.1, 121.1, 118.1, 115.5, 113.1, 107.6, 106.9, 102.9, 59.9, 44.9, 14.3; ESI-MS m/z: 460.1 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{6 }\)[M+H]\(^{+}\): 460.1509; Found 460.1496.
Ethyl 3-(4-hydroxy-3-(hydroxymethyl)benzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4t)
Light green solid; Yield: 81.3 %; Mp: 202–204 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, DMSO) \(\delta \) 10.36 (br, 1H, OH), 9.76 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 9.56 (t, \(J\) = 5.9 Hz,1H, NH), 8.86 (s, 1H, indolizine-H-2), 8.54 (d, \(J\) = 4.0 Hz, 1H, ArH), 7.89 (d, \(J\) = 2.2 Hz,1H, ArH), 7.82–7.75 (m, 1H, ArH), 7.72 (s, 1H, indolizine-H-8), 7.68 (dd, \(J\) = 7.5, 1.9 Hz, 1H, indolizine-H-6), 7.67–7.63 (m, 1H, ArH), 7.39 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.33-7.26 (m, 1H, ArH), 6.96 (d, \(J\) = 8.4 Hz, 1H, ArH), 5.17 (br, 1H, OH), 4.64 (d, \(J\) = 5.8 Hz, 2H, Ar-CH\(_{2})\), 4.56 (s, 2H, CH\(_{2})\), 4.34 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.34 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, DMSO) \(\delta \) 184.0, 164.4, 163.0, 158.3, 158.2, 148.9, 137.5, 136.7, 132.1, 129.5, 129.0, 128.6, 128.3, 126.9, 123.1, 122.2, 121.1, 118.2, 114.3, 113.2, 106.8, 59.9, 57.8, 44.9, 14.3; ESI-MS m/z: 474.2 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{23}\)N\(_{3}\)O\(_{6}\): C, 65.75; H, 4.91; N, 8.93; Found: C, 65.95; H, 4.90; N, 8.87.
Ethyl 3-(3,4-dichlorobenzoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4u)
Yellow solid; Yield: 56.5 %; Mp: 192–195 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.92 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.87 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.0 Hz, 1H, ArH), 7.99 (s, 1H, NH), 7.92 (s, 1H, ArH), 7.83 (s, 1H, indolizine-H-8), 7.75 (t, \(J\) = 7.5 Hz, 1H, ArH), 7.69–7.60 (m, 3H, ArH + indolizine-H-6), 7.38 (d, \(J\) = 7.5 Hz, 1H, ArH), 7.31–7.24 (m, 1H, ArH), 4.82 (d, \(J\) = 3.6 Hz, 2H, Ar-CH\(_{2})\), 4.42 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.45 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 183.0, 165.0, 163.8, 155.6, 149.1, 139.3, 139.1, 137.4, 136.5, 133.4, 133.3, 131.0, 130.8, 129.2, 128.3, 122.9, 122.8, 122.6, 118.3, 114.1, 109.1, 60.8, 44.9, 14.7; ESI-MS m/z: 497.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{19}\)Cl\(_{2}\)N\(_{3}\)O\(_{4}\): C, 60.32; H, 3.85; N, 8.43; Found: C, 60.50; H, 3.86; N, 8.47.
Ethyl 3-(benzo[d][1,3]dioxole-5-carbonyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4v)
Off white solid; Yield: 67.4 %; Mp: 174–176 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.87 (d, \(J\) = 7.2 Hz, 1H, indolizine-H-5), 8.86 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.6 Hz, 1H, ArH), 8.01–7.91 (m, 1H, NH), 7.89 (s, 1H, indolizine-H-8), 7.76 (t, \(J\) = 7.8 Hz, 1H, ArH), 7.57 (d, \(J\) = 7.6 Hz, 1H, ArH), 7.45 (d, \(J\) = 7.7 Hz, 1H, ArH), 7.40 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-6), 7.36 (s, 1H, ArH), 7.30 (d, \(J\) = 5.8 Hz, 1H, ArH), 6.94 (d, \(J\) = 7.8 Hz, 1H, ArH), 6.10 (s, 2H, OCH\(_{2})\), 4.83 (d, \(J\) = 3.8 Hz, 2H, Ar-CH\(_{2})\), 4.43 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.45 (t, \(J\) = 7.0 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 184.2, 165.2, 163.9, 156.0, 151.1, 149.2, 148.1, 138.5, 137.1, 133.7, 132.5, 128.9, 128.6, 125.2, 123.4, 122.7, 122.4, 118.2, 113.4, 109.4, 108.2, 108.1, 102.0, 60.5, 45.0, 14.6; ESI-MS m/z: 472.1 [M+H]\(^{+}\); Anal. Calcd. for C\(_{26}\)H\(_{21}\)N\(_{3}\)O\(_{6}\): C, 66.00; H, 4.57; N, 8.75; Found: C, 66.24; H, 4.49; N, 8.91.
Ethyl 3-(furan-2-carbonyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4w)
Yellow solid; Yield: 27.0 %; Mp: 164–166 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.03 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.86 (s, 1H, indolizine-H-2), 8.59 (d, \(J\) = 4.7Hz, 1H), 8.55 (s, 1H, indolizine-H-8), 7.92 (s, 1H, NH), 7.72 (t, \(J\) = 6.8 Hz, 2H, ArH), 7.59-7.53 (m, 1H, ArH), 7.40 (d, \(J\) = 3.5 Hz, 1H, ArH), 7.35 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.29–7.21 (m, 1H, ArH), 6.63 (d, \(J\) = 5.2 Hz, 1H, ArH), 4.81 (d, \(J\) = 4.5 Hz, 2H, Ar-CH\(_{2})\), 4.45 (q, 7.1 Hz, 2H, CH\(_{2})\), 1.48 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 171.4, 165.2, 164.1, 155.8, 153.4, 149.3, 146.3, 138.5, 137.1, 132.8, 129.3, 128.2, 122.8, 122.4, 118.3, 118.2, 113.7, 112.5, 109.1, 60.7, 45.0, 14.8; ESI-MS m/z: 418.0 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{23}\)H\(_{19}\)N\(_{3}\)O\(_{5}\) [M+H]\(^{+}\):418.1403; Found 418.1404
Ethyl 7-(pyridin-2-ylmethylcarbamoyl)-3-(thiophene-2-carbonyl)indolizine-1-carboxylate (4x)
Yellow solid; Yield: 63.6 %; Mp: 168–170 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.83 (d, \(J\) = 6.4 Hz, 1H, indolizine-H-5), 8.84 (s, 1H, indolizine-H-2), 8.59 (s, 1H, indolizine-H-8), 8.18 (s, 1H, NH), 7.96–7.80 (m, 2H, ArH), 7.70 (d, \(J\) = 4.3 Hz, 2H, ArH), 7.54 (d, \(J\) = 5.9 Hz, 1H, ArH), 7.35 (d, \(J\) = 6.9 Hz, 1H, ArH), 7.30–7.13 (m, 2H, ArH), 4.81 (s, 2H, Ar-CH\(_{2})\), 4.43 (q, \(J\) = 6.7 Hz, 2H, CH\(_{2})\), 1.46 (t, \(J\) = 6.7 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 176.8, 165.1, 164.0, 155.8, 149.3, 144.1, 138.6, 137.1, 132.8, 132.6, 129.0, 128.1, 127.6, 123.2, 122.8, 122.4, 118.3, 113.6, 108.7, 60.7, 45.0, 14.7; ESI-MS m/z: 433.9 [M+H]\(^{+}\); Anal. Calcd. for C\(_{23}\)H\(_{19}\)N\(_{3}\)O\(_{4}\)S: C, 63.52; H, 4.67; N, 9.69; Found: C, 63.73; H, 4.42; N, 9.69.
Ethyl 3-(1-naphthoyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4y)
Yellow solid; Yield: 36.4 %; Mp: 163–165 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.19 (d, \(J\) = 7.3 Hz, 1H, indolizine-H-5), 8.88 (s, 1H, indolizine-H-2), 8.60 (d, \(J\) = 4.6 Hz, 1H, ArH), 8.12 (d, \(J\) = 8.0 Hz, 1H, ArH), 8.02 (d, \(J\) = 8.2 Hz, 1H, ArH), 8.00–7.91 (m, 2H, ArH), 7.76–7.65 (m, 3H, ArH), 7.64 (s, 1H, indolizine-H-8), 7.60-7.49 (m, 3H, ArH), 7.36 (d, \(J\) = 7.9 Hz, 1H, ArH), 7.28–7.22 (m, 1H, ArH), 4.83 (d, \(J\) = 4.6 Hz, 2H, Ar-CH\(_{2})\), 4.35 (q, \(J\) = 7.1 Hz, 2H, CH\(_{2})\), 1.38 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 187.2, 165.1, 163.9, 155.8, 149.3, 139.0, 137.3, 137.1, 134.0, 133.1, 131.1, 131.0, 130.1, 129.4, 128.6, 127.4, 127.0, 126.7, 125.6, 124.8, 124.7, 122.8, 122.4, 118.2, 114.0, 108.7, 60.6, 45.0, 14.6; ESI-MS m/z: 478.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{29}\)H\(_{23}\)N\(_{3}\)O\(_{4}\): C, 72.77; H, 4.84; N, 8.92; Found: C, 72.94; H, 4.85; N, 8.80.
Ethyl 7-(pyridin-2-ylmethylcarbamoyl)-3-(pyrrolidine-1-carbonyl)indolizine-1-carboxylate (4z-1)
Brown solid; Yield: 48.7,%; Mp: 68–70 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.41 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.73 (s, 1H, indolizine-H-2), 8.55 (d, \(J\) = 4.1 Hz, 1H, ArH), 7.99 (s, 1H, NH), 7.73–7.62 (m, 2H, ArH), 7.33 (dd, \(J\) = 7.5, 1.9 Hz, 2H, ArH), 7.24–7.16 (m, 1H, ArH), 4.77 (d, \(J\) = 4.9 Hz, 2H, Ar-CH\(_{2})\), 4.38 (q, \(J\) = 7.1 Hz, 2H, CH\(_{2})\), 3.87-3.58 (br, 4H, pyrrolidine-2CH\(_{2})\), 2.05–s1.90 (br, 4H, pyrrolidine-2CH\(_{2})\), 1.43 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 165.6, 164.3, 161.0, 156.2, 149.2, 137.0, 136.1, 130.1, 128.5, 122.6, 122.3, 121.4, 119.4, 118.2, 111.7, 106.7, 60.3, 49.6, 47.0, 45.0, 26.6, 24.1, 14.7; ESI-MS m/z: 421.2 [M+H]\(^{+}\).
Ethyl 3-(morpholine-4-carbonyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4z-2)
Brown solid; Yield: 56.9 %; Mp: 90-92 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 8.95 (d, \(J\) = 7.4 Hz, 1H, indolizine-H-5), 8.73 (s, 1H, indolizine-H-2), 8.57 (d, \(J\) = 4.4 Hz, 1H, ArH), 7.96 (s, 1H, NH), 7.70 (t, \(J\) = 7.7 Hz, 1H, ArH), 7.48 (s, 1H, indolizine-H-8), 7.42–7.32 (m, 2H, ArH), 7.27 –7.19 (m, 1H, ArH), 4.78 (d, \(J\) = 4.8 Hz, 2H, Ar-CH\(_{2})\), 4.40 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 3.89-3.82 (m, 4H, morpholine-2CH\(_{2})\), 3.81-3.74 (m, 4H, morpholine-2CH\(_{2})\), 1.45 (t, \(J\) = 7.1 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 165.5, 164.3, 161.8, 156.1, 149.2, 137.1, 136.4, 130.2, 127.8, 122.7, 122.4, 121.0, 118.3, 117.8, 112.1, 106.8, 67.1, 60.4, 45.7, 45.0, 14.7; ESI-MS m/z: 437.0 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{23}\)H\(_{24}\)N\(_{4}\)O\(_{5 }\)[M+H]\(^{+}\):437.1825; Found 437.1814.
Ethyl 3-(piperazine-1-carbonyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4z-3)
Light yellow solid; Yield: 52.6 %; Mp: 72–74 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CD\(_{3}\)OD) \(\delta \) 8.73 (d, \(J\) = 6.8 Hz, 1H, indolizine-H-5), 8.62 (s, 1H, indolizine-H-2), 8.50 (s, 1H, NH), 7.82 (t, \(J\) = 7.0 Hz, 1H, ArH), 7.47 (d, \(J\) = 9.4 Hz, 2H, ArH), 7.32 (s, 1H, indolizine-H-8), 7.27 (d, \(J\) = 6.8 Hz, 1H, indolizine-H-6), 4.72 (s, 2H, Ar-CH\(_{2})\), 4.35 (q, \(J\) = 6.5 Hz, 2H, CH\(_{2})\), 3.90–3.77 (br, 4H, piperazine-2CH\(_{2})\), 3.06–2.90 (br, 4H, piperazine-2CH\(_{2})\), 1.39 (t, \(J\) = 6.4 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CD\(_{3}\)OD) \(\delta \) 167.9, 165.45, 163.1, 159.1, 149.9, 139.0, 137.3, 130.81, 128.5, 123.9, 123.2, 121.7, 119.9, 119.5, 112.4, 107.7, 61.5, 46.3, 46.2, 15.0; ESI-MS m/z: 436.1 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{23}\)H\(_{25}\)N\(_{5}\)O\(_{4 }\)[M+H]\(^{+}\): 436.1985; Found 436.1974.
Ethyl 3-(4-methyl-1,4-diazepane-1-carbonyl)-7-(pyridin-2-ylmethylcarbamoyl)indolizine-1-carboxylate (4z-4)
Brown solid; Yield: 45.0 %; Mp: 62–64 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.01 (d, \(J\) = 5.9 Hz, 1H, indolizine-H-5), 8.71 (s, 1H, indolizine-H-2), 8.57 (s, 1H, ArH), 8.01 (s, 1H, NH), 7.69 (t, \(J\) = 7.3 Hz, 1H, ArH), 7.51 (s, 1H, indolizine-H-8), 7.34 (d, \(J\) = 7.4 Hz, 2H, ArH), 7.23 (t, \(J\) = 5.1 Hz, 1H, ArH), 4.78 (d, \(J\) = 3.5 Hz, 2H, Ar-CH\(_{2}\)), 4.39 (q, \(J\) = 6.9 Hz, 2H, CH\(_{2}\)), 3.98–3.77 (br, 4H, 1-methyl-1,4-diazepane-2CH\(_{2}\)), 2.89–2.73 (br, 2H, 1-methyl-1,4-diazepane-CH\(_{2}\)), 2.70–2.57 (br, 2H, 1-methyl-1,4-diazepane-CH\(_{2}\)), 2.43 (s, 3H, NCH\(_{3}\)), 2.12–1.98 (br, 2H, 1-methyl-1,4-diazepane-CH\(_{2}\)), 1.44 (t, \(J\) = 6.9 Hz, 3H, CH\(_{3}\)); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 165.6, 164.3, 162.8, 156.2, 149.2, 137.0, 136.1, 130.0, 127.9, 122.7, 122.3, 121.0, 118.7, 118.2, 111.8, 106.6, 60.4, 58.2, 46.7, 45.0, 27.7, 14.7; ESI-MS m/z: 464.2 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+}\)) m/z Calcd. for C\(_{25}\)H\(_{29}\)N\(_{5}\)O\(_{4 }\)[M+H]\(^{+}\):464.2298; Found 464.2287.
Ethyl 3-benzoyl-8-(pyridin- 2-ylmethylcarbamoyl)indolizine-1-carboxylate (6)
Brown solid; Yield: 65.6 %;Mp: 126–129 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}\)) \(\delta \) 9.99 (d, \(J\) = 6.9 Hz, 1H, indolizine-H-5), 8.48 (d, \(J\) = 4.6 Hz, 1H, ArH), 7.81 (s, 1H, indolizine-H-2), 7.79 (s, 2H, ArH), 7.68 (t, \(J\) = 7.6 Hz, 1H, ArH), 7.58 (d, \(J\) = 7.0 Hz, 2H, ArH + indolizine-H-7), 7.51 (t, \(J\) = 7.1 Hz, 3H, ArH), 7.38 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.19 (t, \(J\) = 6.0 Hz, 1H, ArH), 7.06 (t, \(J\) = 6.9 Hz, 1H, indolizine-H-6), 4.82 (d, \(J\) = 4.3 Hz, 2H, Ar-CH\(_{2})\), 4.16 (q, \(J\) = 7.0 Hz, 2H, CH\(_{2})\), 1.23 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}\)) \(\delta \) 185.7, 167.2, 163.3, 156.0, 148.9, 139.8, 136.9, 135.1, 131.8, 130.2, 129.9, 129.3, 129.1, 128.6, 127.3, 122.7, 122.5, 122.4, 114.4, 108.2, 60.4, 45.0, 14.4; ESI-MS m/z: 428.0 [M+H]\(^{+}\); Anal. Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{4}\): C, 69.99; H, 4.92; N, 9.65; Found: C, 70.25; H, 4.95; N, 9.83.
Ethyl 1-benzoyl-6-(pyridin- 2-ylmethylcarbamoyl)indolizine-3-carboxylate (7)
Yellow solid; Yield: 75.3 %; Mp: 151–153 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 10.16 (s, 1H, indolizine-H-5), 8.64 (d, \(J\) = 9.3 Hz, 1H, indolizine-H-8), 8.60 (d, \(J\) = 9.3 Hz, 1H, indolizine-H-7), 7.88–7.81 (m, 5H, ArH), 7.72 (t, \(J\) = 7.1 Hz, 1H, ArH), 7.60 (t, \(J\) = 7.2 Hz, 1H, ArH), 7.54 (t, \(J\) = 7.3 Hz, 2H, ArH), 7.37 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.27–7.22 (m, 1H, ArH), 4.82 (d, \(J\) = 4.6 Hz, 2H, Ar-CH\(_{2})\), 4.41 (q, \(J\) = 7.2 Hz, 2H, CH\(_{2})\), 1.41 (t, \(J\) = 7.2 Hz, 3H, CH\(_{3})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 190.6, 164.9, 161.1, 156.0, 149.3, 140.2, 140.1, 137.1, 131.8, 129.2, 128.6, 128.6, 127.1, 125.2, 123.6, 122.7, 122.4, 120.4, 116.0, 113.6, 60.9, 45.0, 14.7; ESI-MS m/z: 428.1 [M+H]\(^{+}\); HRMS (ESI-TOF\(^{+})\) m/z Calcd. for C\(_{25}\)H\(_{21}\)N\(_{3}\)O\(_{4 }\)[M+H]\(^{+}\):428.1610; Found 428.1599.
3-Benzoyl-N-(pyridin-2-ylmethyl)imidazo[1,2-a]pyridine-7-carboxamide (8)
Off white solid; Yield: 87.2 %; Mp: 195–197 \(^{\circ }\)C; \(^{1}\)H NMR (400 MHz, CDCl\(_{3}) \delta \) 9.80 (d, \(J\) = 7.2 Hz, 1H, imidazo [1,2-a]pyridine-H-5), 8.61 (d, \(J\) = 4.5 Hz, 1H, ArH), 8.36 (s, 1H, imidazo[1,2-a]pyridine-H-2), 8.31 (s, 1H, imidazo [1,2-a]pyridine-H-8), 8.12 (s, 1H, NH), 7.96–7.88 (m, 2H, ArH), 7.74 (td, \(J\) = 7.7, 1.6 Hz, 1H, ArH), 7.71–7.62 (m, 2H, ArH), 7.57 (t, \(J\) = 7.5 Hz, 2H, ArH), 7.37 (d, \(J\) = 7.8 Hz, 1H, ArH), 7.30-7.26 (m, 2H, ArH), 4.83 (d, \(J\) = 4.5 Hz, 2H, Ar-CH\(_{2})\); \(^{13}\)C NMR (100 MHz, CDCl\(_{3}) \delta \) 185.2, 164.8, 155.4, 149.3, 148.5, 146.5, 139.2, 137.2, 135.1, 132.6, 129.1, 129.0, 128.9, 124.3, 122.9, 122.4, 116.6, 113.6, 44.9; ESI-MS m/z: 357.0 [M+H]\(^{+}\).
Docking procedures
The crystal structure of ElonginC in a complex with ElonginB and VIF BC-box (PDB code: 3DCG) was used to predict the binding models of chemicals bound to ElonginC [26, 27]. The binding poses of compounds with ElnginC were predicted with the GOLD 3.0.1 program (Cambridge Crystallographic Data Center, Cambridge, UK, 2006). All the water molecules were removed from the main protein. Then, the protein file was prepared using the Clean Protein Tool in DiscoveryStudio 2.5 package (Accelrys, San Diego, CA). All the ligands were drawn in ChemDraw Ultra 11.0 (Cambridge Soft Corporation, Cambridge, 2008) and then prepared using Prepare Ligands Module in Discovery Studio 2.5. In this process, the compounds were protonated at pH 7.4 and their low-energy conformations were generated. In the docking simulation, VIF “Val142-Leu145” peptide was used for defining binding site, and the radius of the binding site was defind as 8 Ǻ , sufficient to cover all the binding pockets. The ChemScore fitness function was utilized to evaluate the binding ability of various conformations. The genetic algorithm parameters were set as follows: number of operations 100,000, migrate 0, mutate 95, crossover 95, other parameters were set as default. Each ligand was docked 50 times and each pose was ranked according to its ChemScore fitness function.
Biology
Preliminary screening for VIF inhibitors
293T cells were seeded into 24-well plates in DMEM supplemented with 10% PBS and incubated overnight at 37\(^\circ \)C. Then, cells were transfected with pEYFP-A3G plus HxB\(_{2}\)VIF-cmyc or a control plasmid VR1012 using Lipofectamine 2000 (Invitrogen) as recommended by the manufacturer. Cells were then cultured with different samples (final concentrationat 50 \(\upmu \)M) or DMSO. Cells were harvest at 48 h post transfection. YFP fluorescence was monitored using a Fluoroskan Ascent FL plate reader (Thermo Scientific) with excitation at 485 nm and emission at 538 nm. YFP expression transfected with pEYFP-A3G plus VR1012 and treated with DMSO was set to 100 %.
Antiviral assay
Exponentially growing H9 cells were incubated with wild-type HIV-1 virus at 37\(^{\circ }\) c for 3 h. After removal of the inocula and three extensive washings, cells were seeded into 96-well plates at a density of 1 \(\times \) 10\(^{4}\)/well in 100 L of RPMI 1640. A 100 \(\upmu \)L aliquot of compound solutions (ranging from 0 to 200 \(\upmu \)M) were added and the cells were incubated for 7 days at 37\(^\circ \)C under 5 % CO\(_{2}\). At day 7, p24 levels in each wells were evaluated using a p24 ELISA kit (Perklin Elmer, Norwalk, CT, USA). The IC\(_{50 }\) (concentration inhibiting HIV-1 by 50 %) value was determined by plotting the p24 antigen contents against the drug concentration. To test the cytotoxicity of the compounds, exponentially growing uninfected H9 cells were identically seeded and cultured to that in the HIV antiviral assay. At day 7, cytotoxicity was evaluated with a 3-(4, 5-dimethylthiazol -2-yl)-2, 5-diphenyltetrazolium bromide colorimetric assay (MTT assay). CC\(_{50}\) represents the compound concentration that decreases the cell viability by 50 %.
References
U.S. Food and Drug Administration (2012) Drugs used in the treatment of HIV infection. Silver Spring, MD. http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm. Accessed 17 August 2012
D’Aquila RT, Schapiro JM, Brun-Vezinet F, Clotet B, Conway B, Demeter LM, Grant RM, Johnson VA, Kuritzkes DR, Loveday C, Shafer RW, Richman DD (2003) Drug resistance mutations in HIV-1. Topics in HIV medicine. The international AIDS society, USA 11:92–96
Richman DD, Morton SC, Wrin T, Hellmann N, Berry S, Shapiro MF, Bozzette SA (2004) The prevalence of antiretroviral drug resistance in the United States. AIDS 18:1393–1401
Ross L, Lim ML, Liao Q, Wine B, Rodriguez AE, Weinberg W, Shaefer M (2007) Prevalence of antiretroviral drug resistance and resistance-associated mutations in antiretroviral therapy-naive HIV-infected individuals from 40 United States cities. HIV Clin Trials 8:1–8. doi:10.1310/hct0801-1
Adamson CS, Freed EO (2010) Novel approaches to inhibiting HIV-1 replication. Antivir Res 85:119–141. doi:10.1016/j.antiviral.2009.09.009
Greene WC, Debyser Z, Ikeda Y, Freed EO, Stephens E, Yonemoto W, Buckheit RW, Este JA, Cihlar T (2008) Novel targets for HIV therapy. Antivir Res 80:251–265. doi:10.1016/j.antiviral.2008.08.003
Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH (2003) DNA deamination mediates innate immunity to retroviral infection. Cell 113:803–809
Lecossier D, Bouchonnet F, Clavel F, Hance AJ (2003) Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300:1112. doi:10.1126/science.1083338
Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D (2003) Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99–103. doi:10.1038/nature01709
Izumi T, Shirakawa K, Takaori-Kondo A (2008) Cytidine deaminases as a weapon against retroviruses and a new target for antiviral therapy. Mini Rev Med Chem 8:231–238
Takumi K, Katsumi M, Shuhei K, Masaki M, Daisuke K, Ronald CC, Joan WC, Keiichil N (2004) VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Gene Dev 18:3055–3065. doi:10.1101/gad.1252404
Mehle A, Thomas ER, Rajendran KS, Gabuzda D (2006) A zinc-binding region in Vif binds Cul5 and determines cullin selection. J Biol Chem 281:17259–17265. doi:10.1074/jbc.M602413200
Fujita M, Akari H, Sakurai A, Yoshida A, Chiba T, Tanaka K, Strebel K, Adachi A (2004) Expression of HIV-1 accessory protein Vif is controlled uniquely to be low and optimal by proteasome degradation. Microbes Infect/Institut Pasteur 6:791–798. doi:10.1016/j.micinf.2004.04.011
Xiao Z, Ehrlich E, Yu Y, Luo K, Wang T, Tian C, Yu XF (2006) Assembly of HIV-1 Vif-Cul5 E3 ubiquitin ligase through a novel zinc-binding domain-stabilized hydrophobic interface in Vif. Virology 349:290–299. doi:10.1016/j.virol.2006.02.002
Stanley BJ, Ehrlich ES, Short L, Yu Y, Xiao Z, Yu XF, Xiong Y (2008) Structural insight into the human immunodeficiency virus Vif SOCS box and its role in human E3 ubiquitin ligase assembly. J Virol 82:8656–8663. doi:10.1128/JVI.00767-08
Nathans R, Cao H, Sharova N, Ali A, Sharkey M, Stranska R, Stevenson M, Rana TM (2008) Small-molecule inhibition of HIV-1 Vif. Nat Biotech 26:1187–1192. doi:10.1038/nbt.1496
Cen S, Peng ZG, Li XY, Li ZR, Ma J, Wang YM, Fan B, You XF, Wang YP, Liu F, Shao RG, Zhao LX, Yu L, Jiang JD (2010) Small molecular compounds inhibit HIV-1 replication through specifically stabilizing APOBEC3G. J Biol Chem 285:16546–16552. doi:10.1074/jbc.M109.085308
Fan G, Li Z, Shen S, Zeng Y, Yang Y, Xu M, Bruhn T, Bruhn H, Morschhauser J, Bringmann G, Lin W (2010) Baculiferins A-O, O-sulfated pyrrole alkaloids with anti-HIV-1 activity, from the Chinese marine sponge Iotrochota baculifera. Bioorg Med Chem 18:5466–5474. doi:10.1016/j.bmc.2010.06.052
Idrees Mohammed MKP, Jiang X, Sharova N, Singh G, Stevenson M, Rana TM (2012) SAR and lead optimization of an HIV-1 VIF-APOBEC3G axis inhibitor. ACS Med Chem Lett 3:465–469. doi:10.1021/ml300037k
Zuo T, Liu D, Lv W, Wang X, Wang J, Lv M, Huang W, Wu J, Zhang H, Jin H, Zhang L, Kong W, Yu X (2012) Small-molecule inhibition of human immunodeficiency virus type 1 replication by targeting the interaction between Vif and ElonginC. J Virol 86:5497–5507. doi:10.1128/JVI.06957-11
Lv W, Liu Z, Jin H, Yu X, Zhang L (2007) Three-dimensional structure of HIV-1 VIF constructed by comparative modeling and the function characterization analyzed by molecular dynamics simulation. Org Biomol Chem 5:617–626. doi:10.1039/b612050d
Vemula V, Vurukonda S, Bairi C (2011) Indolizine derivatives: recent advances and potential pharmacological activities. Int J Pharm Sci Rev Res 11:159–163
Singh GS, Mmatli EE (2011) Recent progress in synthesis and bioactivity studies of indolizines. Eur J Med Chem 46:5237–5257. doi:10.1016/j.ejmech.2011.08.042
Wang BX, Liu WW, He T, Hu HW (2006) Oxidant promoted 1, 3-dipolar cycloaddition of pyridinium ylides to chalcones for preparation of 1-benzoyl-2-arylindolizines. Chin J Chem 24:279–281
Gomez O, Salgado-Zamor H, Reyes A, Campos ME (2011) A revised approach to the synthesis of 3-acyl imidazo [1,2-a] pyridines. Heterocycl Comm 16:99–104
Stanley BJ, Ehrlich ES, Short L, Yu Y, Xiao Z, Yu XF, Xiong Y (2008) Structural insight into the human immunodeficiency virus Vif SOCS box and its role in human E3 ubiquitin ligase assembly. J Virol 82:8656–8663
Marcsisin SR, Engen JR (2010) Molecular insight into the conformational dynamics of the Elongin BC complex and its interaction with HIV-1 Vif. J Mol Biol 402:892–904
Acknowledgments
This work was supported by the National Natural Science Foundation of China (20972009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, W., Zuo, T., Jin, H. et al. Design, synthesis and biological evaluation of indolizine derivatives as HIV-1 VIF–ElonginC interaction inhibitors. Mol Divers 17, 221–243 (2013). https://doi.org/10.1007/s11030-013-9424-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9424-3