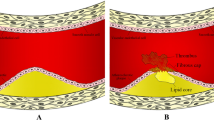
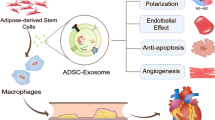
Abstract
Cardiovascular disease (CVD) has a high incidence and low cure rate worldwide, and atherosclerosis (AS) is the main factor inducing cardiovascular disease, of which lipid deposition in the vessel wall is the main marker of AS. Currently, although statins can be used to lower lipids and low-density lipoprotein (LDL) in AS, the cure rate for AS remains low. Therefore, there is an urgent need to develop new therapeutic approaches, and stem cells are now widely studied, while stem cells are a class of cell types that always maintain the ability to differentiate and can differentiate to form other cells and tissues, and stem cell transplantation techniques have shown efficacy in the treatment of other diseases. With the establishment of cellular therapies and continued research in stem cell technology, stem cells are also being used to address the problem of AS. In this paper, we focus on recent research advances in stem cell therapy for AS and briefly summarize the relevant factors that induce the formation of AS. We mainly discuss the efficacy and application prospects of mesenchymal stem cells (MSCs) for the treatment of AS, in addition to the partial role and potential of exosomes in the treatment of AS. Further, provide new ideas for the clinical application of stem cells.



Similar content being viewed by others
Data availability
This declaration is “not applicable”.
References
Kirwin T, Gomes A, Amin R et al (2021) Mechanisms underlying the therapeutic potential of mesenchymal stem cells in atherosclerosis[J]. Regen Med 16(7):669–682
Benjamin EJ, Blaha MJ, Chiuve SE et al (2017) Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association[J]. Circulation 135(10):e146–e603
Scott J (2004) Pathophysiology and biochemistry of cardiovascular disease[J]. Curr Opin Genet Dev 14(3):271–279
Li J, Xue H, Li T et al (2019) Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE-/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage[J]. Biochem Biophys Res Commun 510(4):565–572
Xing X, Li Z, Yang X et al (2020) Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis[J]. Aging (Albany NY) 12(4):3880–3898
Peloso GM, Natarajan P (2018) Insights from population-based analyses of plasma lipids across the allele frequency spectrum[J]. Curr Opin Genet Dev 50:1–6
Liang J, Li W, Liu H et al (2022) Di’ao Xinxuekang Capsule Improves the Anti-Atherosclerotic Effect of Atorvastatin by Downregulating the SREBP2/PCSK9 Signalling Pathway[J]. Front Pharmacol 13:857092
Liang X, Lin F, Ding Y et al (2021) Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis[J]. Stem Cell Res Ther 12(1):295
Sun Y, Song L, Zhang Y et al (2020) Adipose stem cells from type 2 diabetic mice exhibit therapeutic potential in wound healing[J]. Stem Cell Res Ther 11(1):298
Ulpiano C, Da Silva CL, Monteiro GA (2021) Mesenchymal Stromal Cells (MSCs): A Promising Tool for Cell-Based Angiogenic Therapy[J]. Curr Gene Ther 21(5):382–405
Nakamura Y, Kita S, Tanaka Y et al (2020) Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice[J]. Mol Ther 28(10):2203–2219
Traunmüller F (2018) Atherosclerosis is a vascular stem cell disease caused by insulin[J]. Med Hypotheses 116:22–27
Wang D, Li LK, Dai T et al (2018) Adult Stem Cells in Vascular Remodeling[J]. Theranostics 8(3):815–829
Lusis AJ (2021) A vicious cycle in atherosclerosis[J]. Cell 184(5):1139–1141
Fan Q, Yin X, Rababa’h A et al (2019) Absence of gravin-mediated signaling inhibits development of high-fat diet-induced hyperlipidemia and atherosclerosis[J]. Am J Physiol Heart Circ Physiol 317(4):H793-h810
Song P, Fang Z, Wang H et al (2020) Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study[J]. Lancet Glob Health 8(5):e721–e729
Hassanisaber H, Rouleau L, Faucheux N (2019) Effect of BMP-9 on endothelial cells and its role in atherosclerosis[J]. Front Biosci (Landmark edition) 24(6):994–1023
Takafuji Y, Hori M, Mizuno T et al (2019) Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice[J]. Cardiovasc Res 115(6):1041–1051
Georgiopoulos G, Mavraganis G, Delialis D et al (2022) Carotid ultrasonography improves residual risk stratification in guidelines-defined high cardiovascular risk patients[J]. Eur J Prev Cardiol. https://doi.org/10.1093/eurheartj/ehac544.2317
Gorzelak-Pabiś P, Pawlos A, Broncel M et al (2022) Expression of anti and pro-inflammatory genes in human endothelial cells activated by 25-hydroxycholesterol : a comparison of rivaroxaban and dabigatran[J]. Clin Exp Pharmacol Physiol. https://doi.org/10.1111/1440-1681.13668
Drinkall S, Lawrence C, Ossola B et al (2022) The two pore potassium channel THIK-1 regulates NLRP3 inflammasome activation[J]. Glia 70(7):1301–1316
Liu D, Liu J, Zhang D et al (2022) Advances in relationship between cell senescence and atherosclerosis[J] Zhejiang da xue xue bao Yi xue ban = journal of Zhejiang university. Med Sci 51(1):95–101
Li X, Yang Y, Wang Z et al (2021) Targeting non-coding RNAs in unstable atherosclerotic plaques: mechanism, regulation, possibilities, and limitations[J]. Int J Biol Sci 17(13):3413–3427
Ridker P, Rane M (2021) Interleukin-6 Signaling and anti-interleukin-6 therapeutics in cardiovascular disease[J]. Circ Res 128(11):1728–1746
Bielas H, Meister-Langraf R, Schmid J et al (2022) Relationship between a self-reported history of depression and persistent elevation in c-reactive protein after myocardial infarction[J]. J Clin Med 11(9):2322
Libby P, Buring JE, Badimon L et al (2019) Atherosclerosis[J]. Nat Rev Dis Primers 5(1):56
Borén J, Chapman MJ, Krauss RM et al (2020) Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel[J]. Eur Heart J 41(24):2313–2330
Yao T, Lu W, Ke J et al (2022) Residual risk of coronary atherosclerotic heart disease and severity of coronary atherosclerosis assessed by ApoB and LDL-C in participants With statin treatment: a retrospective cohort study[J]. Front Endocrinol 13:865863
Liu C, Wu J, Jia H et al (2022) Oncostatin M promotes the ox-LDL-induced activation of NLRP3 inflammasomes via the NF-κB pathway in THP-1 macrophages and promotes the progression of atherosclerosis[J]. Annals of translational medicine 10(8):456
Singh S, Siva B, Ravichandiran V (2022) Advanced Glycation End Products: key player of the pathogenesis of atherosclerosis[J]. Glycoconj J 39(4):547–563
Aguilar-Ballester M, Herrero-Cervera A, Vinué Á et al (2020) Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis[J]. Nutrients 12(7):2021
Bäck M, Yurdagul A Jr, Tabas I et al (2019) Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities[J]. Nat Rev Cardiol 16(7):389–406
Nasser MI, Zhu S, Huang H et al (2020) Macrophages: First guards in the prevention of cardiovascular diseases[J]. Life Sci 250:117559
Horckmans M, Ring L, Duchene J et al (2017) Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype[J]. Eur Heart J 38(3):187–197
Ren Q, Xie X, Zhao C et al (2022) 2,2’,4,4’-Tetrabromodiphenyl ether (PBDE 47) selectively stimulates proatherogenic PPARγ signatures in human THP-1 macrophages to contribute to foam cell formation[J]. Chem Res Toxicol 35(6):1023–1035
Ye Z, Guo H, Wang L et al (2022) GALNT4 primes monocytes adhesion and transmigration by regulating O-Glycosylation of PSGL-1 in atherosclerosis[J]. J Mol Cell Cardiol 165:54–63
Choi YY, Kim A, Seong KM (2021) Chronic radiation exposure aggravates atherosclerosis by stimulating neutrophil infiltration[J]. Int J Radiat Biol 97(9):1270–1281
Yang Y, Wang D, Zhang C et al (2022) Piezo1 mediates endothelial atherogenic inflammatory responses via regulation of YAP/TAZ activation[J]. Hum Cell 35(1):51–62
Takehara Y (2022) Clinical application of 4D Flow MR Imaging for the Abdominal Aorta[J]. Magn Reson Med Sci 21(2):354–364
Lin L, Zhang MX, Zhang L et al (2021) Autophagy, pyroptosis, and ferroptosis: new regulatory mechanisms for atherosclerosis[J]. Front Cell Dev Biol 9:809955
Poznyak A, Grechko AV, Poggio P et al (2020) The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation [J]. Int J Mol Sci 21(5):1835
Mauersberger C, Hinterdobler J, Schunkert H et al (2021) Where the Action Is-Leukocyte Recruitment in Atherosclerosis[J]. Front Cardiovasc Med 8:813984
Jia J, Wang Y, Huang R et al (2022) Protein disulfide-isomerase A3 knockdown attenuates oxidized low-density lipoprotein-induced oxidative stress, inflammation and endothelial dysfunction in human umbilical vein endothelial cells by downregulating activating transcription factor 2[J]. Bioengineered 13(1):1436–1446
Bobryshev YV, Orekhov AN, Chistiakov DA (2015) Vascular stem/progenitor cells: current status of the problem[J]. Cell Tissue Res 362(1):1–7
Wang X, Wang R, Jiang L et al (2022) Endothelial repair by stem and progenitor cells[J]. J Mol Cell Cardiol 163:133–146
Matsushima K, Yang D, Oppenheim JJ (2022) Interleukin-8: An evolving chemokine[J]. Cytokine 153:155828
Zhuo X, Bu H, Hu K et al (2021) Differences in the reaction of hyperlipidemia on different endothelial progenitor cells based on sex[J]. Biomed Rep 15(2):64
Hu Q, Dong X, Zhang K et al (2022) Fluid shear stress ameliorates prehypertension-associated decline in endothelium-reparative potential of early endothelial progenitor cells[J]. J Cardiovasc Transl Res 15(5):1049–1063
Fang J, Huang X, Han X et al (2020) Endothelial progenitor cells promote viability and nerve regenerative ability of mesenchymal stem cells through PDGF-BB/PDGFR-β signaling[J]. Aging (Albany NY) 12(1):106–121
Gan F, Liu L, Zhou Q et al (2022) Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis[J]. PLoS ONE 17(1):e0261498
Saito N, Shirado T, Funabashi-Eto H et al (2022) Purification and characterization of human adipose-resident microvascular endothelial progenitor cells[J]. Sci Rep 12(1):1775
Jiang RC, Zheng XY, Yang SL et al (2022) CD146 mediates the anti-apoptotic role of Netrin-1 in endothelial progenitor cells under hypoxic conditions[J]. Mol Med Rep. https://doi.org/10.3892/mmr.2021.12521
Zhang L, Issa Bhaloo S, Chen T et al (2018) Role of Resident Stem Cells in Vessel Formation and Arteriosclerosis[J]. Circ Res 122(11):1608–1624
Yurdagul A Jr (2022) Crosstalk between macrophages and Vascular smooth muscle cells in atherosclerotic plaque stability[J]. Arterioscler Thromb Vasc Biol 42(4):372–380
Wang H, Xing M, Deng W et al (2022) Anti-Sca-1 antibody-functionalized vascular grafts improve vascular regeneration via selective capture of endogenous vascular stem/progenitor cells[J]. Bioact Mater 16:433–450
Zhang N, Luo Y, Zhang H et al (2022) Exosomes derived from mesenchymal stem cells ameliorate the progression of atherosclerosis in ApoE(-/-) Mice via FENDRR[J]. Cardiovasc Toxicol 22(6):528–544
Pang QM, Chen SY, Fu SP et al (2022) Regulatory role of mesenchymal stem cells on secondary inflammation in spinal cord injury[J]. J Inflamm Res 15:573–593
Egea V, Megens RTA, Santovito D et al (2022) Properties and fate of human mesenchymal stem cells upon miRNA let-7f-promoted recruitment to atherosclerotic plaques[J]. Cardiovasc Res 119(1):155–166
Frodermann V, Van Duijn J, Van Pel M et al (2015) Mesenchymal stem cells reduce murine atherosclerosis development[J]. Sci Rep 5:15559
Sharma M, Schlegel MP, Afonso MS et al (2020) Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression[J]. Circ Res 127(3):335–353
Hong R, Wang Z, Sui A et al (2019) Gingival mesenchymal stem cells attenuate pro-inflammatory macrophages stimulated with oxidized low-density lipoprotein and modulate lipid metabolism[J]. Arch Oral Biol 98:92–98
Zhang X, Huang F, Li W et al (2018) Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate Atherosclerosis[J]. Front Immunol 9:878
Li JZ, Cao TH, Han JC et al (2019) Comparison of adipose- and bone marrow-derived stem cells in protecting against ox-LDL-induced inflammation in M1-macrophage-derived foam cells[J]. Mol Med Rep 19(4):2660–2670
Lin Y, Zhu W, Chen X (2020) The involving progress of MSCs based therapy in atherosclerosis[J]. Stem Cell Res Ther 11(1):216
Zhang D, Lin Y, Li Y et al (2021) Mesenchymal stem cells enhance Treg immunosuppressive function at the fetal-maternal interface[J]. J Reprod Immunol 148:103366
Mu Y, Xu W, Liu J et al (2022) Mesenchymal stem cells moderate experimental autoimmune uveitis by dynamic regulating Th17 and Breg cells response[J]. J Tissue Eng Regen Med 16(1):26–35
Zhang Z, Li Z, Wang Y et al (2021) PDGF-BB/SA/Dex injectable hydrogels accelerate BMSC-mediated functional full thickness skin wound repair by promoting angiogenesis[J]. J Mater Chem B 9(31):6176–6189
Li C, An Y, Sun Y et al (2022) Adipose mesenchymal stem cell-derived exosomes promote wound healing through the WNT/β-catenin Signaling pathway in dermal fibroblasts[J]. Stem Cell Rev Rep 16(6):2059–2073
Ba Z, Shi S, Huang N et al (2022) Mesenchymal stem cells after the proprocessing of tanshinone IIA attenuate cognitive deficits and oxidative stress injury in an amyloid β-peptide (25–35)-induced rodent model of Alzheimer’s disease[J]. NeuroReport 33(2):61–71
Su G, Lei X, Wang Z, et al. Mesenchymal stem cell-derived exosomes affect macrophage phenotype: a cell-free strategy for the treatment of skeletal muscle disorders[J]. Curr Mol Med, 2022.
Wang ZX, Wang CQ, Li XY et al (2015) Mesenchymal stem cells alleviate atherosclerosis by elevating number and function of CD4(+)CD25 (+)FOXP3 (+) regulatory T-cells and inhibiting macrophage foam cell formation[J]. Mol Cell Biochem 400(1–2):163–172
Otsuka F, Yasuda S, Noguchi T et al (2016) Pathology of coronary atherosclerosis and thrombosis[J]. Cardiovasc Diagn Ther 6(4):396–408
Wang SS, Hu SW, Zhang QH et al (2015) Mesenchymal Stem Cells Stabilize Atherosclerotic Vulnerable Plaque by Anti-Inflammatory Properties[J]. PLoS ONE 10(8):e0136026
Lin Y, Liu M, Chen E et al (2021) Bone marrow-derived mesenchymal stem cells microvesicles stabilize atherosclerotic plaques by inhibiting NLRP3-mediated macrophage pyroptosis[J]. Cell Biol Int 45(4):820–830
Cheng G, Wang X, Li Y et al (2017) Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling[J]. Stem Cell Res Ther 8(1):34
Wang S, Tong M, Hu S et al (2018) The bioactive substance secreted by MSC retards mouse aortic vascular smooth muscle cells calcification[J]. Biomed Res Int 2018:6053567
Kharlamov AN, Tyurnina AE, Veselova VS et al (2015) Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial[J]. Nanoscale 7(17):8003–8015
Li Q, Sun W, Wang X et al (2015) Skin-derived mesenchymal stem cells alleviate atherosclerosis via modulating macrophage function[J]. Stem Cells Transl Med 4(11):1294–1301
Wei X, Sun G, Zhao X et al (2019) Human amnion mesenchymal stem cells attenuate atherosclerosis by modulating macrophage function to reduce immune response[J]. Int J Mol Med 44(4):1425–1435
Li Y, Shi G, Han Y et al (2021) Therapeutic potential of human umbilical cord mesenchymal stem cells on aortic atherosclerotic plaque in a high-fat diet rabbit model[J]. Stem Cell Res Ther 12(1):407
Li Q, Wang Y, Li H et al (2014) Ox-LDL influences peripheral Th17/Treg balance by modulating Treg apoptosis and Th17 proliferation in atherosclerotic cerebral infarction[J]. Cell Physiol Biochem 33(6):1849–1862
Khosravi M, Bidmeshkipour A, Cohen JL et al (2018) Induction of CD4(+)CD25(+)FOXP3(+) regulatory T cells by mesenchymal stem cells is associated with modulation of ubiquitination factors and TSDR demethylation[J]. Stem Cell Res Ther 9(1):273
Durham AL, Speer MY, Scatena M et al (2018) Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness[J]. Cardiovasc Res 114(4):590–600
Xie C, Ouyang L, Chen J et al (2019) The emerging role of mesenchymal stem cells in vascular calcification[J]. Stem Cells Int 2019:2875189
Wang S, Hu S, Wang J et al (2018) Conditioned medium from bone marrow-derived mesenchymal stem cells inhibits vascular calcification through blockade of the BMP2-Smad1/5/8 signaling pathway[J]. Stem Cell Res Ther 9(1):160
Wang Y, Xie Y, Zhang A et al (2019) Exosomes: an emerging factor in atherosclerosis[J]. Biomed Pharmacother 115:108951
Poller W, Dimmeler S, Heymans S et al (2018) Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives[J]. Eur Heart J 39(29):2704–2716
Jansen F, Yang X, Proebsting S et al (2014) MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease[J]. J Am Heart Assoc 3(6):e001249
Raju S, Fish JE, Howe KL (2020) MicroRNAs as sentinels and protagonists of carotid artery thromboembolism[J]. Clin Sci (Lond) 134(2):169–192
Jiang H, Toscano JF, Song SS et al (2019) Differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antiangiogenesis[J]. Sci Rep 9(1):19429
Liu Y, Li Q, Hosen MR et al (2019) Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles[J]. Circ Res 124(4):575–587
Wang Z, Zhang J, Zhang S et al (2019) MiR-30e and miR-92a are related to atherosclerosis by targeting ABCA1[J]. Mol Med Rep 19(4):3298–3304
Hajibabaie F, Kouhpayeh S, Mirian M et al (2020) MicroRNAs as the actors in the atherosclerosis scenario[J]. J Physiol Biochem 76(1):1–12
Mallia A, Gianazza E, Zoanni B et al (2020) Proteomics of extracellular vesicles: update on their composition, biological roles and potential use as diagnostic tools in atherosclerotic cardiovascular diseases[J]. Diagnostics (Basel) 10(10):843
Bellin G, Gardin C, Ferroni L et al (2019) Exosome in cardiovascular diseases: a complex world full of hope[J]. Cells 8(2):166
Guo D, Xu Y, Ding J et al (2020) Roles and clinical applications of exosomes in cardiovascular disease[J]. Biomed Res Int 2020:5424281
Boulanger CM, Loyer X, Rautou PE et al (2017) Extracellular vesicles in coronary artery disease[J]. Nat Rev Cardiol 14(5):259–272
Kozakai M, Narita Y, Yamawaki-Ogata A et al (2022) Alternative therapeutic strategy for existing aortic aneurysms using mesenchymal stem cell-derived exosomes[J]. Expert Opin Biol Ther 22(1):95–104
Piovesan A, Antonaros F, Vitale L et al (2019) Human protein-coding genes and gene feature statistics in 2019[J]. BMC Res Notes 12(1):315
Yu C, Tang W, Lu R et al (2021) Human Adipose-derived mesenchymal stem cells promote lymphocyte apoptosis and alleviate atherosclerosis via miR-125b-1-3p/BCL11B signal axis[J]. Ann Palliat Med 10(2):2123–2133
Lin F, Zhang S, Liu X et al (2021) Mouse bone marrow derived mesenchymal stem cells-secreted exosomal microRNA-125b-5p suppresses atherosclerotic plaque formation via inhibiting Map4k4[J]. Life Sci 274:119249
Ma J, Chen L, Zhu X et al (2021) Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis[J]. Acta Biochim Biophys Sin (Shanghai) 53(9):1227–1236
Gao H, Yu Z, Li Y et al (2021) miR-100-5p in human umbilical cord mesenchymal stem cell-derived exosomes mediates eosinophilic inflammation to alleviate atherosclerosis via the FZD5/Wnt/β-catenin pathway[J]. Acta Biochim Biophys Sin (Shanghai) 53(9):1166–1176
Sakic A, Chaabane C, Ambartsumian N et al (2022) Neutralization of S100A4 induces stabilization of atherosclerotic plaques: role of smooth muscle cells[J]. Cardiovasc Res 118(1):141–155
Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A et al (2022) Pathophysiology of Atherosclerosis[J]. Int J Mol Sci 23(6):3346
Yang J, Cao RY, Gao R et al (2017) Physical exercise Is a potential “medicine” for Atherosclerosis[J]. Adv Exp Med Biol 999:269–286
Funding
This work was supported by the National Key Research and Development Project (2019YFA0801601) and the National Natural Science Foundation of China (81971087).
Author information
Authors and Affiliations
Contributions
All authors contributed the writing and conception of the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This declaration is “not applicable”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Gu, T., He, S. et al. Development of stem cell therapy for atherosclerosis. Mol Cell Biochem 479, 779–791 (2024). https://doi.org/10.1007/s11010-023-04762-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04762-8