Abstract
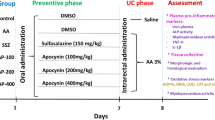
Targeting oxidative stress and inflammation by novel dietary compounds of natural origin convincingly appears to be one of the most important therapeutic strategies to keep inflammatory bowel diseases (IBD) such as ulcerative colitis disease in remission. It is imperative to investigate naturally occuring plant-derived dietary phytochemicals that are receiving attention for their therapeutic benefits to overcome the debilitating conditions of IBD. In the present study, the effect of nerolidol (NRD), a monocyclic sesquiterpene found in German Chamomile tea, was investigated in acetic acid-induced colitis model in Wistar rats. NRD was orally administered at a dose of 50 mg/kg/day either for 3 days before or 30 min after induction of IBD for 7 days, after intrarectal administration of acetic acid. The body weight, macroscopic, and microscopic analyses of the colon in different experimental groups were observed on days 0, 2, 4, and 7. Acetic acid caused significant reduction in body weight and induced macroscopic and microscopic ulcer along with a significant decline of antioxidants, concomitant to increased malondialdehyde (MDA), a marker of lipid peroxidation, and myeloperoxidase (MPO) activity, a marker of neutrophil activation. Treatment with NRD significantly improved IBD-induced reduction in body weight, improved histology, inhibited MDA formation, and restored antioxidants along with reduced MPO activity. Acetic acid also induced the release of pro-inflammatory cytokines and increased calprotectin, released by neutrophils under inflammatory conditions. NRD treatment significantly reduced calprotectin and pro-inflammatory cytokines. NRD treatment showed potential to improve disease activity and inhibit oxidative stress, lipid peroxidation, and inflammation along with histological preservation of the colon tissues.





Similar content being viewed by others
Data availability
The manuscript data are well archived with the principal investigator, Prof. Salim MA Bastaki. The manuscript data will be available to the editors upon reasonable request from Prof. Salim Bastaki.
References
Malik TA (2015) Inflammatory bowel disease: historical perspective, epidemiology, and risk factors. Surg Clin N Am 95(1105–1122):v
Stepaniuk P, Bernstein CN, Targownik LE, Singh H (2015) Characterization of inflammatory bowel disease in elderly patients: a review of epidemiology, current practices and outcomes of current management strategies. Can J Gastroenterol Hepatol 29:327–333
Tenailleau QM, Lanier C, Gower-Rousseau C, Cuny D, Deram A, Occelli F (2020) Crohn’s disease and environmental contamination: Current challenges and perspectives in exposure evaluation. Environ Pollut 263:114599
Baumgart DC, Carding SR (2007) Inflammatory bowel disease: cause and immunobiology. Lancet 369:1627–1640
Marafini I, Sedda S, Dinallo V, Monteleone G (2019) Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther 19(11):1207–1217
Leppkes M, Neurath MF (2020) Cytokines in inflammatory bowel diseases—update 2020. Pharmacol Res 158:104835
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94:329–354
Moura FA, Goulart MOF, Campos SBG, da Paz Martins AS (2020) The close interplay of nitro-oxidative stress, advanced glycation end products and inflammation in inflammatory bowel diseases. Curr Med Chem 27:2059–2076
Hazel K, O’Connor A (2020) Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. https://doi.org/10.1177/2040622319899297
Chapman TP, Gomes CF, Louis E, Colombel JF (2020) Review Article: withdrawal of 5-aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther 52(1):73–84
Vachon A, Scott FI (2020) The treatment approach to inflammatory bowel disease in 2020. Curr Opin Gastroenterol 36:247–256
Algieri F, Rodriguez-Nogales A, Rodriguez-Cabezas ME, Risco S, Ocete MA, Galvez J (2015) Botanical drugs as an emerging strategy in inflammatory bowel disease: a review. Mediators Inflamm 2015:179616
Triantafyllidi A, Xanthos T, Papalois A, Triantafillidis JK (2015) Herbal and plant therapy in patients with inflammatory bowel disease. Ann Gastroenterol 28:210–220
Ganji-Arjenaki M, Rafieian-Kopaei M (2019) Phytotherapies in inflammatory bowel disease. J Res Med Sci 24:42
Venkataraman B, Ojha S, Belur PD, Bhongade B, Raj V, Collin PD et al (2020) Phytochemical drug candidates for the modulation of peroxisome proliferator-activated receptor γ in inflammatory bowel diseases. Phytother Res 34(7):1530–1549
Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P (2020) Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med 9(5):1273
Ambrose T, Simmons A (2019) Cannabis, cannabinoids, and the endocannabinoid system—is there therapeutic potential for inflammatory bowel disease? J Crohns Colitis 13(4):525–535
Chan WK, Tan LT, Chan KG, Lee LH, Goh BH (2016) Nerolidol: a sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 21(5):529
Sun S, Du GJ, Qi LW, Williams S, Wang CZ, Yuan CS (2010) Hydrophobic constituents and their potential anticancer activities from Devil’s Club (Oplopanax horridus Miq.). J Ethnopharmacol 132:280–285
Dall’Acqua S, Peron G, Ferrari S, Gandin V, Bramucci M, Quassinti L et al (2017) Phytochemical investigations and antiproliferative secondary metabolites from Thymus alternans growing in Slovakia. Pharm Biol 55:1162–1170
Zhou Y, Zeng L, Liu X, Gui J, Mei X, Fu X et al (2017) Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem 231:78–86
Alves RF, Nascimento AMD, Nogueira JMF (2005) Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal Chim Acta 546:11–21
Javed H, Azimullah S, Abul Khair SB, Ojha S, Haque ME (2016) Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci 17:58
Baldissera MD, Souza CF, Grando TH, Dolci GS, Cossetin LF, Moreira KL et al (2017) Nerolidol-loaded nanospheres prevent hepatic oxidative stress of mice infected by Trypanosoma evansi. Parasitology 144:148–157
Iqubal A, Sumit S, Ansari MA, Najmi AK, Syed MA, Ali J et al (2019) Nerolidol attenuates cyclophosphamide-induced cardiac inflammation, apoptosis and fibrosis in Swiss albino mice. Eur J Pharmacol 863:172666
Thapa D, Richardson AJ, Zweifel B, Wallace RJ, Gratz SW (2019) Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human colon cancer cells. J Food Sci 84:1979–1985
Fonsêca DV, Salgado PR, de Carvalho FL, Salvadori MG, Penha AR, Leite FC et al (2016) Nerolidol exhibits antinociceptive and anti-inflammatory activity: involvement of the GABAergic system and proinflammatory cytokines. Fundam Clin Pharmacol 30:14–22
Ferreira MOG, Leite LLR, de Lima IS, Barreto HM, Nunes LCC, Ribeiro AB et al (2016) Chitosan hydrogel in combination with nerolidol for healing wounds. Carbohydr Polym 152:409–418
Zhang L, Sun D, Bao Y, Shi Y, Cui Y, Guo M (2017) Nerolidol protects against LPS-induced acute kidney injury via inhibiting TLR4/NF-κB signaling. Phytother Res 31:459–465
Ni YL, Shen HT, Su CH, Chen WY, Huang-Liu R, Chen CJ et al (2019) Nerolidol suppresses the inflammatory response during lipopolysaccharide-induced acute lung injury via the modulation of antioxidant enzymes and the AMPK/Nrf-2/HO-1 pathway. Oxid Med Cell Longev 2019:9605980
Iqubal A, Syed MA, Najmi AK, Ali J, Haque SE (2020) Ameliorative effect of nerolidol on cyclophosphamide-induced gonadal toxicity in Swiss Albino mice: biochemical-, histological- and immunohistochemical-based evidences. Andrologia 52:e13535
Yang H, Wang Q, Han L, Yang X, Zhao W, Lyu L et al (2020) Nerolidol inhibits the LOX-1/IL-1β signaling to protect against the Aspergillus fumigatus keratitis inflammation damage to the cornea. Int Immunopharmacol 80:106118
Klopell FC, Lemos M, Sousa JP, Comunello E, Maistro EL, Bastos JK et al (2007) Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z Naturforsch C J Biosci 62:537–542
Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N (2018) The beneficial effects of nerolidol and hesperidin on surgically induced endometriosis in a rat model. Gynecol Endocrinol 34:975–980
Kaur D, Pahwa P, Goel RK (2016) Protective effect of nerolidol against pentylenetetrazol-induced kindling, oxidative stress and associated behavioral comorbidities in mice. Neurochem Res 41:2859–2867
Wallace JL, Keenan CM (1990) An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol 258:G527–G534
Lestringant GG, Masouyé I, Frossard PM, Adeghate E, Galadari IH (1997) Co-existence of leukoderma with features of Dowling-Degos disease: reticulate acropigmentation of Kitamura spectrum in five unrelated patients. Dermatology 195:337–343
Lestringant GG, Frossard PM, Adeghate E, Qayed KI (1997) Mal de Meleda: a report of four cases from the United Arab Emirates. Pediatr Dermatol 14:186–191
Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W (1995) Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 182:1281–1290
Medicherla K, Sahu BD, Kuncha M, Kumar JM, Sudhakar G, Sistla R (2015) Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct 6:2984–2995
de Santana Souza MT, Teixeira DF, de Oliveira JP, Oliveira AS, Quintans-Júnior LJ, Correa CB et al (2017) Protective effect of carvacrol on acetic acid-induced colitis. Biomed Pharmacother 96:313–319
Bastaki SM, Adeghate E, Amir N, Ojha S, Oz M (2018) Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am J Transl Res 10:4210–4222
Kalra J, Lingaraju MC, Mathesh K, Kumar D, Parida S, Singh TU et al (2018) Betulinic acid alleviates dextran sulfate sodium-induced colitis and visceral pain in mice. Naunyn Schmiedebergs Arch Pharmacol 391:285–297
Jia Z, Xu C, Shen J, Xia T, Yang J, He Y (2015) The natural compound celastrol inhibits necroptosis and alleviates ulcerative colitis in mice. Int Immunopharmacol 29:552–559
Santos FA, Silva RM, Campos AR, De Araújo RP, Lima Júnior RC, Rao VS (2004) 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem Toxicol 42:579–584
MacPherson BR, Pfeiffer CJ (1978) Experimental production of diffuse colitis in rats. Digestion 17:135–150
Warren BF (1994) Another model of acetic acid-induced colitis in the rat. Aliment Pharmacol Ther 8:659–660
Strober W, Fuss IJ (2011) Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1756–1767
Santana MT, Cercato LM, Oliveira JP, Camargo EA (2017) Medicinal plants in the treatment of colitis: evidence from preclinical studies. Planta Med 83:588–614
Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME et al (2016) Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev 2016:5276130
Orlando RC (2010) The integrity of the esophageal mucosa Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol 24:873–882
Sørbye H, Svanes K (1994) The role of blood flow in gastric mucosal defence, damage and healing. Dig Dis 12:305–317
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625
Chaudhary G, Mahajan UB, Goyal SN, Ojha S, Patil CR, Subramanya SB (2017) Protective effect of Lagerstroemia speciosa against dextran sulfate sodium-induced ulcerative colitis in C57BL/6 mice. Am J Transl Res 9:1792–1800
Balmus IM, Ciobica A, Trifan A, Stanciu C (2016) The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: clinical aspects and animal models. Saudi J Gastroenterol 22:3–17
Alzoghaibi MA, Al Mofleh IA, Al-Jebreen AM (2007) Lipid peroxides in patients with inflammatory bowel disease. Saudi J Gastroenterol 13:187–190
Bastaki SMA, Al Ahmed MM, Al Zaabi A, Amir N, Adeghate E (2016) Effect of turmeric on colon histology, body weight, ulcer, IL-23, MPO and glutathione in acetic-acid-induced inflammatory bowel disease in rats. BMC Complement Altern Med 16:72
Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK (2008) Role of cytokines in inflammatory bowel disease. World J Gastroenterol 14:4280–4288
Sartor RB (1994) Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology 106:533–539
Fizur NMM, Azimullah A, Laham F, Saeed T, Goyal SN, Adeghate E et al (2020) α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Funct. https://doi.org/10.1039/C9FO00530G
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87:1344–1350
Mullane KM, Kraemer R, Smith B (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 14:157–167
Wéra O, Lancellotti P, Oury C (2016) The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 5(12):118
Chatzikonstantinou M, Konstantopoulos P, Stergiopoulos S, Kontzoglou K, Verikokos C, Perrea D et al (2016) Calprotectin as a diagnostic tool for inflammatory bowel diseases. Biomed Rep 5:403–407
Author information
Authors and Affiliations
Contributions
SMAB and SO have conceptualized the study. SMAB designed the experiments, and interpreted the data. NA has performed test treatments, animal care, experiments, and biochemical estimations. NA carried out the statistical analysis of all the data collected and archived all the data. EA performed the histopathological studies and interpreted the observations. SMBA and SO wrote the first draft of manuscript and SMBA significantly edited the manuscript. SO and SMBA have revised and submitted the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors are grateful for the financial support from the United Arab Emirates University and for providing facilities to conduct the experiments. The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bastaki, S.M.A., Amir, N., Adeghate, E. et al. Nerolidol, a sesquiterpene, attenuates oxidative stress and inflammation in acetic acid-induced colitis in rats. Mol Cell Biochem 476, 3497–3512 (2021). https://doi.org/10.1007/s11010-021-04094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04094-5