Abstract
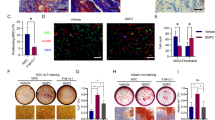
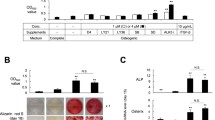
We hypothesized that a crosstalk between osteoblast and fibroblast (FB) exists, which contributes to bone as a dynamic tissue. Cell-free supernatants were harvested from fibroblast cultures and later subject pre-osteoblasts to investigate there capacity to modulate cell viability and differentiation mechanisms, reporting the possible involvement of Shh signaling as a paracrine mechanism. By exploring immunoblotting technology, we have shown that FB-released factors interfere with osteoblast metabolism by up-regulating the phosphorylation of FAK and Rac-1 proteins at the early stage and later contribute to osteoblast differentiation by up-modulating alkaline phosphatase (ALP) and in vitro mineralization. We also found that Shh signaling was not required during osteoblastic differentiation promoted by the FB-released factors as well as MAPK-ERK phosphorylation, while pre-osteoblast cultures subjected to osteogenic medium (O.M.) require downstream transducers of Shh, such as Patched and Gli-1, and MAPK-ERK. Altogether, our results indicate for the first time a possible mechanism involved in the crosstalk between fibroblasts and osteoblasts, as it was possible to observe trophic factors released by fibroblasts interfering decisively in osteoblast metabolism in a Shh-independent manner. This study collaborates the body of work that indicates paracrine signaling molecules participate in the crosstalk among bone-resident cells and explains, at least partially, the biological mechanisms responsible for bone tissue dynamism, opening new avenues to understand etiologies of bone diseases.




Similar content being viewed by others
References
Ap Kusumbe, Sk Ramasamy, Adams Rh (2014) Coupling Of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507:323–328. doi:10.1038/Nature13145
Sk Ramasamy, Ap Kusumbe, Adams Rh (2015) Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol 25:148–157. doi:10.1016/J.Tcb.2014.11.007
Funck-Brentano T, Cohen-Solal M (2011) Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor Rev 22:91–97. doi:10.1016/J.Cytogfr.2011.04.003
Brotto M, Bonewald L (2015) Bone and muscle: interactions beyond mechanical. Bone 80:109–114. doi:10.1016/J.Bone.2015.02.010
Gemini-Piperni S, Milani R, Bertazzo S, Al Et (2014) Kinome profiling of osteoblasts on hydroxyapatite opens new avenues on biomaterial cell signaling. Biotechnol Bioeng 111:1900–1905. doi:10.1002/Bit.25246
Ducy P, Schinke T, Karsenty G (2000) The osteoblast: a sophisticated fibroblast under central surveillance. Science 289:1501–1504
Lee B, Thirunavukkarasu K, Zhou L, Al Et (1997) Missense mutations abolishing dna binding of the osteoblast-specific transcription factor Osf2/Cbfa1 in cleidocranial dysplasia. Nat Genet 16:307–310. doi:10.1038/Ng0797-307
Ducy P, Desbois C, Boyce B, Al Et (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452. doi:10.1038/382448a0
Ducy P, Zhang R, Geoffroy V, Al Et (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Ac Carreira, Gg Alves, Wf Zambuzzi, Al Et (2014) Bone morphogenetic proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys 561:64–73. doi:10.1016/J.Abb.2014.07.011
Gs Baht, Silkstone D, Nadesan P, Al Et (2014) Activation of Hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J Orthop Res 32:581–586. doi:10.1002/Jor.22562
Krishnan L, Lb Priddy, Esancy C, Al Et (2017) Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose Bmp-2. Acta Biomater 49:101–112. doi:10.1016/J.Actbio.2016.12.012
Lee J, Sk Madhurakkat Perikamana, Ahmad T, Al Et (2017) Controlled retention of Bmp-2-derived peptide on nanofibers based on mussel-inspired adhesion for bone formation. Tissue Eng Part A. doi:10.1089/Ten.Tea.2016.0363
Kazmers Nh, Ja Mckenzie, Ts Shen, Al Et (2015) Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone 81:524–532. doi:10.1016/J.Bone.2015.09.002
Wf Zambuzzi, Cl Yano, Adm Cavagis, Al Et (2009) Ascorbate-induced osteoblast differentiation recruits distinct Mmp-inhibitors: reck And Timp-2. Mol Cell Biochem 322:143–150. doi:10.1007/S11010-008-9951-X
Wf Zambuzzi, Bruni-Cardoso A, Jm Granjeiro, Al Et (2009) On the road to understanding of the osteoblast adhesion: cytoskeleton organization is rearranged by distinct signaling pathways. J Cell Biochem 108:134–144. doi:10.1002/Jcb.22236
Cavagis A, Takamori E, Granjeiro J, Al Et (2014) Tnfalpha contributes for attenuating both Y397fak And Y416src phosphorylations in osteoblasts. Oral Dis 20:780–786. doi:10.1111/Odi.12202
Milani R, Ferreira CV, Jm Granjeiro, Al Et (2010) Phosphoproteome reveals an atlas of protein signaling networks during osteoblast adhesion. J Cell Biochem 109:957–966. doi:10.1002/Jcb.22479
Wf Zambuzzi, Milani R, Teti A (2010) Expanding the role of Src and protein-tyrosine phosphatases balance in modulating osteoblast metabolism: lessons from mice. Biochimie 92:327–332. doi:10.1016/J.Biochi.2010.01.002
Wf Zambuzzi, Ferreira CV, Jm Granjeiro, Aoyama H (2011) Biological behavior of pre-osteoblasts on natural hydroxyapatite: a study of signaling molecules from attachment to differentiation. J Biomed Mater Res A 97:193–200. doi:10.1002/Jbm.A.32933
Gvo Fernandes, Adm Cavagis, Ferreira CV, Al Et (2014) Osteoblast adhesion dynamics: a possible role for Ros and Lmw-Ptp. J Cell Biochem 115:1063–1069. doi:10.1002/Jcb.24691
Sun Z, Ss Guo, Fassler R (2016) Integrin-mediated mechanotransduction. J Cell Biol 215:445–456. doi:10.1083/Jcb.201609037
Ohtsuka S, Ogawa S, Wakamatsu E, Abe R (2016) Cell cycle arrest caused by Mek/Erk signaling is a mechanism for suppressing growth of antigen-hyperstimulated effector T cells. Int Immunol 28:547–557. doi:10.1093/Intimm/Dxw037
Rutault K, Ca Hazzalin, Lc Mahadevan (2001) Combinations of Erk and P38 Mapk inhibitors ablate tumor necrosis factor-alpha (Tnf-Alpha) Mrna induction. Evidence for selective destabilization of Tnf-Alpha transcripts. J Biol Chem 276:6666–6674. doi:10.1074/Jbc.M005486200
Liu X, Zeng L, Zhao Z, Al Et (2017) Pbmc activation via the Erk and Stat signaling pathways enhances the anti-tumor activity of Staphylococcal enterotoxin A. Mol Cell Biochem. doi:10.1007/S11010-017-3038-5
Guo J-P, Li X-G (2017) Galectin-7 promotes the invasiveness of human oral squamous cell carcinoma cells via activation of Erk and Jnk signaling. Oncol Lett 13:1919–1924. doi:10.3892/Ol.2017.5649
Gao F, Li F, Miao Y, Al Et (2017) Involvement of the Mek-Erk/P38-Creb/C-Fos signaling pathway in Kir channel inhibition-induced rat retinal muller cell gliosis. Sci Rep 7:1480. doi:10.1038/S41598-017-01557-Y
Mc Moorer, Hebert C, Re Tomlinson, Al Et (2017) Defective signaling, osteoblastogenesis and bone remodeling in a mouse model of connexin 43 C-terminal truncation. J Cell Sci 130:531–540. doi:10.1242/Jcs.197285
Wang M, Shu Z-J, Wang Y, Peng W (2017) Stachydrine hydrochloride inhibits proliferation and induces apoptosis of breast cancer cells via inhibition of Akt and Erk pathways. Am J Transl Res 9:1834–1844
Acknowledgements
The authors are grateful to FAPESP (#2015/00581-9; #2014/22689-3) and CNPq (#477452/2012-4) for the financial support. W.F.Z. is a PQ-2 fellow from CNPq (#301966/2015-0).
Funding
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (grants #2015/00581-9; #2014/22689-3) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (grant #477452/2012-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare to have no conflict of interest with the materials used in the present study.
Rights and permissions
About this article
Cite this article
da Costa Fernandes, C.J., do Nascimento, A.S., da Silva, R.A. et al. Fibroblast contributes for osteoblastic phenotype in a MAPK-ERK and sonic hedgehog signaling-independent manner. Mol Cell Biochem 436, 111–117 (2017). https://doi.org/10.1007/s11010-017-3083-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3083-0