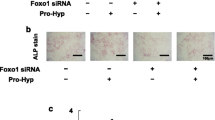
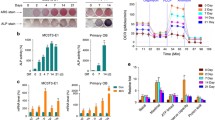
Abstract
The bone formation executed by osteoblasts represents an interesting research field both for basic and applied investigations. The goal of this work was to evaluate the molecular mechanisms involved during osteoblast differentiation in vitro. Accordingly, we demonstrated that, during the osteoblastic differentiation, TIMP-2 and RECK presented differential expressions, where RECK expression was downregulated from the 14th day in contrast with an increase in TIMP-2. Concomitantly, our results showed a temporal regulation of two major signaling cascades during osteoblast differentiation: proliferation cascades in which RECK, PI3 K, and GSK-3β play a pivotal role and latter, differentiation cascades with participation of Ras, Rho, Rac-1, PKCα/β, and TIMP-2. Furthermore, we observed that phosphorylation level of paxillin was downregulated while FAK125 remained unchangeable, but active during extracellular matrix (ECM) remodeling. Concluding, our results provide evidences that RECK and TIMP-2 are involved in the control of ECM remodeling in distinct phases of osteoblast differentiation by modulating MMP activities and a multitude of signaling proteins governs these events.





Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- ECM:
-
Extracellular matrix
- MMP:
-
Matrix metalloproteinase
- MT1-MMP:
-
MT1-matrix metalloproteinase
- TIMP-2:
-
Tissue inhibitors of metalloproteinases
- RECK:
-
Reversion-inducing-cysteine-rich protein with Kazal Motifs
- FAK:
-
Focal adhesion kinase
- p-NPP:
-
p-Nitrophenyl-phosphate
- FBS:
-
Fetal bovine serum
- DTT:
-
Dithiothreitol
- SDS:
-
Sodium dodecyl sulfate
- PMSF:
-
Phenyl-methyl sulphonyl fluoride-serine-protease enzyme inhibitor
- PVDF:
-
Polyvinylidene fluoride
- PTK:
-
Protein tyrosine kinase
- PTP:
-
Protein tyrosine phosphatase
- cdk:
-
Cyclin-dependent kinase
- Rb:
-
Retinoblastoma
- MAPK:
-
Mitogen-activated protein kinase
- LIMK:
-
LIM kinases
- ATCC:
-
American type culture collection
- PBS:
-
Phosphate-buffered saline
- TBS:
-
Tris-buffered saline
- ECL:
-
Enhanced chemoluminescence
- FGF:
-
Fibroblast growth factor
- PI3K:
-
Phosphoinositide-3 kinase
- GSK-3β:
-
Glycogen synthase kinase 3β
- PKC:
-
Protein kinase C
- MDA:
-
Malondialdehyde
- MPO:
-
N-methyl-2-phenylindole
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- GST:
-
Glutathione-S-transferase
References
Valtieri M, Sorrentino A (2008) The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol 217:296–300. doi:10.1002/jcp.21521
Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J Clin Pathol 61:577–587. doi:10.1136/jcp.2007.048868
Krane SM, Inada M (2008) Matrix metalloproteinases and bone. Bone 43:7–18. doi:10.1016/j.bone.2008.03.020
Accorsi-Mendonça T, Paiva KB, Zambuzzi WF, Cestari TM, Lara VS, Sogayar MC, Taga R, Granjeiro JM (2008) Expression of matrix metalloproteinases-2 and -9 and RECK during alveolar bone regeneration in rat. J Mol Histol 39:201–208. doi:10.1007/s10735-007-9152-z
Hannas AR, Pereira JC, Granjeiro JM, Tjaderhane L (2007) The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65:1–13. doi:10.1080/00016350600963640
Mizutani A, Sugiyama I, Kuno E, Matsunaga S, Tsukagoshi N (2001) Expression of matrix metalloproteinases during ascorbate-induced differentiation of osteoblastic MC3T3-E1 cells. J Bone Miner Res 16:2043–2049. doi:10.1359/jbmr.2001.16.11.2043
Hatori K, Sasano Y, Takahashi I, Kamakura S, Kagayama M, Sasaki K (2004) Osteoblasts and osteocytes express MMP-2 and -8 and TIMP-1, -2, and -3 along with extracellular matrix molecules during appositional bone formation. Anat Rec Discov Mol Cell Evol Biol 277:262–271. doi:10.1002/ar.a.20007
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494. doi:10.1074/jbc.274.31.21491
Geoffroy V, Marty-Morieux C, le Goupil N, Clement-Lacroix P, Terraz C, Frain M, Roux S, Rossert J, de Vernejoul MC (2004) In vivo inhibition of osteoblastic metalloproteinases leads to increased trabecular bone mass. J Bone Miner Res 19:811–822. doi:10.1359/JBMR.040119
Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi XX, Kitaura Y, Takai S, Sasahara RM, Horimoto A, Ikawa Y, Ratzkin BJ, Arakawa T, Noda M (1998) Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci USA 95:13221–13226. doi:10.1073/pnas.95.22.13221
Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M (2001) The membrane-anchored MMP inhibitor RECK is a key regulator of extracelular matrix integrity and angiogenesis. Cell 107:789–800. doi:10.1016/S0092-8674(01)00597-9
Sasahara RM, Brochado SM, Takahashi C, Oh J, Maria-Engler SS, Granjeiro JM, Noda M, Sogayar MC (2002) Transcriptional control of the RECK metastasis/angiogenesis suppressor gene. Cancer Detect Prev 26:435–443. doi:10.1016/S0361-090X(02)00123-X
Clark JC, Thomas DM, Choong PF, Dass CR (2007) RECK-a newly discovered inhibitor of metastasis with prognostic significance in multiple forms of cancer. Cancer Metastasis Rev 26:675–683. doi:10.1007/s10555-007-9093-8
Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, Jo JS, Ryoo HM (1996) Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells. J Cell Biochem 61:609–618. doi:10.1002/(SICI)1097-4644(19960616)61:4<609::AID-JCB15>3.0.CO;2-A
Hitomi K, Torii Y, Tsukagoshi N (1992) Increase in the activity of alkaline phosphatase by l-ascorbic acid 2-phosphate in a human osteoblast cell line, HuO-3N1J. Nutr Sci Vitaminol 38:535–544
Hartree EF (1972) Determination of proteins: a modification of Lowry method that give a linear photometric response. Anal Biochem 48:422–427. doi:10.1016/0003-2697(72)90094-2
Yano CL, Marcondes MC (2005) Cadmium chloride-induced oxidative stress in skeletal muscle cells in vitro. Free Radic Biol Med 39:1378–1384. doi:10.1016/j.freeradbiomed.2005.07.001
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first step in mercapturic acid formation. J Biol Chem 249:7130–7139
Gomes-Marcondes MC, Tisdale MJ (2002) Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett 180:69–74. doi:10.1016/S0304-3835(02)00006-X
Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O’Keefe CL, Matera AG, Xiong Y (1994) Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev 8:2939–2952. doi:10.1101/gad.8.24.2939
Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ (1995) Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol 15:2672–2681
Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL (1995) Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem 270:16995–16999. doi:10.1074/jbc.270.28.16995
Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K (1998) Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393:809–812. doi:10.1038/31735
Ferreira CV, Justo GZ, Souza AC, Queiroz KC, Zambuzzi WF, Aoyama H, Peppelenbosch MP (2006) Natural compounds as a source of protein tyrosine phosphatase inhibitors: application to the rational design of small-molecule derivatives. Biochimie 88:1859–1873. doi:10.1016/j.biochi.2006.08.007
Fornoni A, Cornacchia F, Howard GA, Roos BA, Striker GE, Striker LJ (2001) Cyclosporin A affects extracellular matrix synthesis and degradation by mouse MC3T3-E1 osteoblasts in vitro. Nephrol Dial Transplant 16:500–505. doi:10.1093/ndt/16.3.500
Jiang Y, Goldberg ID, Shi YE (2002) Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 21:2245–2252. doi:10.1038/sj.onc.1205291
Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG (2003) TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114:171–180. doi:10.1016/S0092-8674(03)00551-8
Oh J, Seo DW, Diaz T, Wei B, Ward Y, Ray JM, Morioka Y, Shi S, Kitayama H, Takahashi C, Noda M, Stetler-Stevenson WG (2004) Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res 64:9062–9069. doi:10.1158/0008-5472.CAN-04-1981
Pérez-Martínez L, Jaworski DM (2005) Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci 25:4917–4929. doi:10.1523/JNEUROSCI.5066-04.2005
Oh J, Diaz T, Wei B, Chang H, Noda M, Stetler-Stevenson WG (2006) TIMP-2 upregulates RECK expression via dephosphorylation of paxillin tyrosine residues 31 and 118. Oncogene 25:4230–4234. doi:10.1038/sj.onc.1209444
Ridley AJ, Hall A (1992) The small GTP-binding protein rhoA regulates the assembly of focal adhesions anad actin stress fibers in response to growth factors. Cell 70:389–399. doi:10.1016/0092-8674(92)90163-7
Zhang ZY, Dixon JE (1993) Active site labeling of the Yersinia protein tyrosine phosphatase: the determination of the pKa of the active site cysteine and the function of the conserved histidine 402. Biochemistry 32:9340–9345. doi:10.1021/bi00087a012
Peters GH, Frimurer TM, Olsen OH (1998) Electrostatic evaluation of the signature motif (H/V)CX5R(S/T) in protein-tyrosine phosphatases. Biochemistry 37:5383–5393. doi:10.1021/bi971187i
Acknowledgements
We are grateful to Dr. Claudia L. Soraggi, Ms. Denise B. Ciampi and Rodrigo A. da Silva for the technical support. We thank Prof. Hernandes F. Carvalho for critical suggestions. WFZ is supported by a PhD scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (grant no. 04/14906-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zambuzzi, W.F., Yano, C.L., Cavagis, A.D.M. et al. Ascorbate-induced osteoblast differentiation recruits distinct MMP-inhibitors: RECK and TIMP-2. Mol Cell Biochem 322, 143–150 (2009). https://doi.org/10.1007/s11010-008-9951-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9951-x