Abstract
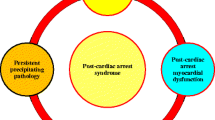
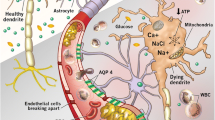
Circulatory arrest (CA) remains a major unresolved public health problem in the United States; the annual incidence of which is ~0.50 to 0.55 per 1000 population. Despite seminal advances in therapeutic approaches over the past several decades, brain injury continues to be the leading cause of morbidity and mortality after CA. In brief, CA typically results in global cerebral ischemia leading to delayed neuronal death in the hippocampal pyramidal cells as well as in the cortical layers. The dynamic changes occurring in neurons after CA are still unclear, and predicting these neurological changes in the brain still remains a difficult issue. It is hypothesized that the “no-flow” period produces a cytotoxic cascade of membrane depolarization, Ca2+ ion influx, glutamate release, acidosis, and resultant activation of lipases, nucleases, and proteases. Furthermore, during reperfusion injury, neuronal death occurs due to the generation of free radicals by interfering with the mitochondrial respiratory chain. The efficacy of many pharmacological agents for CA patients has often been disappointing, reflecting our incomplete understanding of this enigmatic disease. The primary obstacles to the development of a neuroprotective therapy in CA include uncertainties with regard to the precise cause(s) of neuronal dysfunction and what to target. In this review, we summarize our knowledge of the pathophysiology as well as specific cellular changes in brain after CA and revisit the most important neurofunctional, neuroimaging techniques, and serum biomarkers as potent predictors of neurologic outcome in CA patients.





Similar content being viewed by others
References
Mozaffarian D, Benjamin EJ, Go AS et al (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133:e38–e60
Berdowski J, Berg RA, Tijssen JGP, Koster RW (2010) Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 81:1479–1487
Girotra G, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS (2012) Trends in survival after in-hospital cardiac arrest. N Engl J Med 367:1912–1920
Maramattom BV, Wijdicks EF (2005) Postresuscitation encephalopathy. Current views, management, and prognostication. Neurologist 11:234–243
Michenfelder JD, Theye RA (1970) The effects of anesthesia and hypothermia on canine cerebral ATP and lactate during anoxia produced by decapitation. Anesthesiology 33:430–439
Siesjo BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1:155–185
Astrup J, Sorensen PM, Sorensen HR (1981) Oxygen and glucose consumption related to Na+-K+transport in canine brain. Stroke 12:726–730
Caroni P, Carafoli E (1981) Regulation of Ca2+-pumping ATPase of heart sarcolemma by a phosphorylation-dephosphorylation process. J Biol Chem 256:9371–9373
Vaagenes P, Ginsberg M, Ebmeyer U et al (1996) Cerebral resuscitation from cardiac arrest: pathophysiologic mechanisms. Crit Care Med 24:S57–S68
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622
Pin JP, Duvoisin R (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34:1–26
Erecinska M, Silver IA (1992) Relationship between ions and energy metabolism: cerebral calcium movements during ischaemia and subsequent recovery. Can J Physiol Pharmacol 70(Suppl):S190–S193
Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R (1994) Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci 14:3934–3944
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326(Pt 1):1–16
Ushijima K, Miyazaki H, Morioka T (1986) Immunohistochemical localization of glutathione peroxidase in the brain of the rat. Resuscitation 13:97–105
Zaleska MM, Floyd RA (1985) Regional lipid peroxidation in rat brain in vitro: possible role of endogenous iron. Neurochem Res 10:397–410
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14
Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995) Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke 26:1478–1489
Cao W, Carney JM, Duchon A, Floyd RA, Chevion M (1988) Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett 88:233–238
Zamzami N, Marchetti P, Castedo M et al (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377
Piantadosi CA, Zhang J (1996) Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27:327–331
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Bakhle YS (1983) Synthesis and catabolism of cyclo-oxygenase products. Br Med Bull 39:214–218
Moskowitz N, Schook W, Puszkin S (1984) Regulation of endogenous calcium-dependent synaptic membrane phospholipase A2. Brain Res 290:273–279
Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9:119–131
Padosch SA, Popp E, Vogel P, Böttiger BW (2003) Altered protein expression levels of Fas/CD95 and Fas ligand in differentially vulnerable brain areas in rats after global cerebral ischemia. Neurosci Lett 338:247–251
Iadecola C, Zhang F, Xu S, Casey R, Ross ME (1995) Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab 15:378–384
Giulian D, Li J, Leara B, Keenen C (1994) Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int 25:227–233
Barone FC, Hillegass LM, Price WJ et al (1991) Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res 29:336–345
Adams DH, Shaw S (1994) Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet 343:831–836
del Zoppo GJ (1994) Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev 6:47–96
Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC (1998) Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29:1020–1030
Smith CW (1993) Endothelial adhesion molecules and their role in inflammation. Can J Physiol Pharmacol 71:76–87
Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Dávalos A (2003) Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 34:40–46
Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M (1999) NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5:554–559
Klatzo I (1967) Presidental address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol 26:1–14
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276
Chan PH, Schmidley JW, Fishman RA, Longar SM (1984) Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology 34:315–320
Mun-Bryce S, Rosenberg GA (1998) Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab 18:1163–1172
Mayhan WG, Didion SP (1996) Glutamate-induced disruption of the blood-brain barrier in rats. Role of nitric oxide. Stroke 27:965–969
Sampaolo S, Nakagawa Y, Iannotti F, Cervos-Navarro J, Bonavita V (1991) Blood-brain barrier permeability to micromolecules and edema formation in the early phase of incomplete continuous ischemia. Acta Neuropathol 82:107–111
Chalkias A, Xanthos T (2012) Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail Rev 17:117–128
Ibáñez B, Heusch G, Ovize M, Van de Werf F (2015) Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65:1454–1471
Kimmoun A, Novy E, Auchet T, Ducrocq N, Levy B (2015) Hemodynamic consequences of severe lactic acidosis in shock states: from bench to bedside. Crit Care 19:175
Heusch G, Boengler K, Schulz R (2010) Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 105:151–154
Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S (2014) Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care 29:500–511
Omar S, Zedan A, Nugent K (2015) Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. Am J Med Sci 349:80–88
Geppert A, Zorn G, Karth GD et al (2000) Soluble selectins and the systemic inflammatory response syndrome after successful cardiopulmonary resuscitation. Crit Care Med 28:2360–2365
Boland TA, Lee VH, Bleck TP (2015) Stress-induced cardiomyopathy. Crit Care Med 43:686–693
Paur H, Wright PT, Sikkel MB et al (2012) High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 126:697–706
Petito CK, Pulsinelli WA (1984) Delayed neuronal recovery and neuronal death in rat hippocampus following severe cerebral ischemia: possible relationship to abnormalities in neuronal processes. J Cereb Blood Flow Metab 4:194–205
Yamamoto K, Morimoto K, Yanagihara T (1986) Cerebral ischemia in the gerbil: transmission electron microscopic and immunoelectron microscopic investigation. Brain Res 384:1–10
Schmidt-Kastner R, Freund TF (1991) Selective vulnerability of the hippocampus in brain ischemia. Neuroscience 40:599–636
Schmidt-Kastner R, Hossmann KA (1988) Distribution of ischemic neuronal damage in the dorsal hippocampus of rat. Acta Neuropathol 76:411–421
Ito U, Spatz M, Walker JT Jr, Klatzo I (1975) Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol 32:209–223
Hashimoto K, Kikuchi H, Ishikawa M, Kobayashi S (1992) Changes in cerebral energy metabolism and calcium levels in relation to delayed neuronal death after ischemia. Neurosci Lett 137:165–168
Fujioka M, Okuchi K, Sakaki T, Hiramatsu K, Miyamoto S, Iwasaki S (1994) Specific changes in human brain following reperfusion after cardiac arrest. Stroke 25:2091–2095
Panickar KS, Norenberg MD (2005) Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia 50:287–298
Klatzo I, Chui E, Fujiwara K, Spatz M (1980) Resolution of vasogenic brain edema. Adv Neurol 28:359–373
Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA (1990) Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10:1583–1591
Petito CK, Olarte JP, Roberts B, Nowak TS Jr, Pulsinelli WA (1998) Selective glial vulnerability following transient global ischemia in rat brain. J Neuropathol Exp Neurol 57:231–238
Petito CK (1986) Transformation of postischemic perineuronal glial cells. I. Electron microscopic studies. J Cereb Blood Flow Metab 6:616–624
Uchihara T, Tsuchiya K, Nakamura A, Ikeda K (2000) Appearance of tau-2 immunoreactivity in glial cells in human brain with cerebral infarction. Neurosci Lett 286:99–102
Valeriani V, Dewar D, McCulloch J (2000) Quantitative assessment of ischemic pathology in axons, oligodendrocytes, and neurons: attenuation of damage after transient ischemia. J Cereb Blood Flow Metab 20:765–771
Roine RO, Somer H, Kaste M, Viinikka L, Karonen SL (1989) Neurological outcome after out-of-hospital cardiac arrest. Prediction by cerebrospinal fluid enzyme analysis. Arch Neurol 46:753–756
Martens P, Raabe A, Johnsson P (1998) Serum S-100 and neuron-specific enolase for prediction of regaining consciousness after global cerebral ischemia. Stroke 29:2363–2366
Tiainen M, Roine RO, Pettilä V, Takkunen O (2003) Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke 34:2881–2886
Meynaar IA, Oudemans-van Straaten HM, van der Wetering J et al (2003) Serum neuron-specific enolase predicts outcome in post-anoxic coma: a prospective cohort study. Intensive Care Med 29:189–195
Rosen H, Sunnerhagen KS, Herlitz J et al (2001) Serum levels of the brain-derived proteins S-100 and NSE predict long-term outcome after cardiac arrest. Resuscitation 49:183–191
Dauberschmidt R, Zinsmeyer J, Mrochen H, Meyer M (1991) Changes of neuron-specific enolase concentration in plasma after cardiac arrest and resuscitation. Mol Chem Neuropathol 14:237–245
Fogel W, Vieira SR, Nagel F, Brauner JS, Scalco R (1997) Serum neuron-specific enolase as early predictor of outcome after cardiac arrest. Crit Care Med 25:1133–1138
Bottiger BW, Mobes S, Glätzer R, Bauer H, Gries A, Bärtsch P, Motsch J, Martin E (2001) Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation 103:2694–2698
Schoerkhuber W, Kittler H, Sterz F, Behringer W, Holzer M, Frossard M, Spitzauer S, Laggner AN (1999) Time course of serum neuron-specific enolase. A predictor of neurological outcome in patients resuscitated from cardiac arrest. Stroke 30:1598–1603
Marangos PJ, Campbell IC, Schmechel DE, Murphy DL, Goodwin FK (1980) Blood platelets contain a neuron-specific enolase subunit. J Neurochem 34:1254–1258
Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S (2006) Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology 67:203–210
Reisinger J, Höllinger K, Lang W et al (2007) Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur Heart J 28:52–58
Rosen H, Rosengren L, Herlitz J, Blomstrand C (1998) Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke 29:473–477
Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C (2001) Jochum M (2001) S-100b, sE-selectin, and sP-selectin for evaluation of hypoxic brain damage in patients after cardiopulmonary resuscitation: pilot study. World J Surg 25:539–543
Hachimi-Idrissi S, Van der Auwera M, Schiettecatte J, Ebinger G, Michotte Y, Huyghens L (2002) S-100 protein as early predictor of regaining consciousness after out of hospital cardiac arrest. Resuscitation 53:251–257
Zingler VC, Krumm B, Bertsch T, Fassbender K, Pohlmann-Eden B (2003) Early prediction of neurological outcome after cardiopulmonary resuscitation: a multimodal approach combining neurobiochemical and electrophysiological investigations may provide high prognostic certainty in patients after cardiac arrest. Eur Neurol 49:79–84
Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C, Mutschler W, Jochum M (2002) Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med 30:2669–2674
Young GB (2000) The EEG in coma. J Clin Neurophysiol 17:473–485
Jorgensen EO, Holm S (1998) The natural course of neurological recovery following cardiopulmonary resuscitation. Resuscitation 36:111–122
Hirsch LJ, LaRoche SM, Gaspard N et al (2013) American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 30:1–27
Zandbergen EG, Hijdra A, Koelman JH, Hart AA, Vos PE, Verbeek MM, de Haan RJ, PROPAC Study Group (2006) Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 66:62–68
Young GB, Doig G, Ragazzoni A (2005) Anoxic-ischemic encephalopathy: clinical and electrophysiological associations with outcome. Neurocrit Care 2:159–164
Rossetti AO, Carrera E, Oddo M (2012) Early EEG correlates of neuronal injury after brain anoxia. Neurology 78:796–802
Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ (2012) Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med 40:2867–2875
Kawai M, Thapalia U, Verma A (2011) Outcome from therapeutic hypothermia and EEG. J Clin Neurophysiol 28:483–488
Rundgren M, Westhall E, Cronberg T, Rosén I, Friberg H (2010) Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med 38:1838–1844
Oh SH, Park KN, Kim YM, Kim HJ, Youn CS, Kim SH, Choi SP, Kim SC, Shon YM (2013) The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation 84:200–205
Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A (1998) Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet 352:1808–1812
Jorgensen EO, Malchow-Moller A (1981) Natural history of global and critical brain ischaemia. Part II: EEG and neurological signs in patients remaining unconscious after cardiopulmonary resuscitation. Resuscitation 9:155–174
Sorensen K, Thomassen A, Wernberg M (1978) Prognostic significance of alpha frequency EEG rhythm in coma after cardiac arrest. J Neurol Neurosurg Psychiatry 41:840–842
Kaplan PW, Genoud D, Ho TW, Jallon P (1999) Etiology, neurologic correlations, and prognosis in alpha coma. Clin Neurophysiol 110:205–213
Ahmed I (1988) Use of somatosensory evoked responses in the prediction of outcome from coma. Clin EEG Neurosci 19:78–86
Bassetti C, Bomio F, Mathis J, Hess CW (1996) Early prognosis in coma after cardiac arrest: a prospective clinical, electrophysiological, and biochemical study of 60 patients. J Neurol Neurosurg Psychiatry 61:610–615
Rothstein TL (2000) The role of evoked potentials in anoxic-ischemic coma and severe brain trauma. J Clin Neurophysiol 17:486–497
Zandbergen EG, de Haan RJ, Koelman JH, Hijdra A (2000) Prediction of poor outcome in anoxic-ischemic coma. J Clin Neurophysiol 17:498–501
Stelzl T, von Bose MJ, Hogl B, Fuchs HH, Flugel KA (1995) A comparison of the prognostic value of neuron-specific enolase serum levels and somatosensory evoked potentials in 13 reanimated patients. Eur J Emerg Med 2:24–27
Tiainen M, Kovala TT, Takkunen OS, Roine RO (2005) Somatosensory and brainstem auditory evoked potentials in cardiac arrest patients treated with hypothermia. Crit Care Med 33:1736–1740
Robinson LR, Micklesen PJ, Tirschwell DL, Lew HL (2003) Predictive value of somatosensory evoked potentials for awakening from coma. Crit Care Med 31:960–967
Madl C, Grimm G, Kramer L et al (1993) Early prediction of individual outcome after cardiopulmonary resuscitation. Lancet 341:855–858
Castillo M (1998) Imaging of cerebral infarction. Curr Probl Diagn Radiol 27:105–131
Kjos BO, Brant-Zawadzki M, Young RG (1983) Early CT findings of global central nervous system hypoperfusion. Am J Roentgenol 141:1227–1232
Bell BA, Symon L, Branston NM (1985) CBF and time thresholds for the formation of ischemic cerebral edema, and effect of reperfusion in baboons. J Neurosurg 62:31–41
Hatashita S, Hoff JT (1986) Cortical tissue pressure gradients in early ischemic brain edema. J Cereb Blood Flow Metab 6:1–7
Torbey MT, Selim M, Knorr J, Bigelow C, Recht L (2000) Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke 31:2163–2167
Hoeffner EG, Case I, Jain R et al (2004) Cerebral perfusion CT: technique and clinical applications. Radiology 231:632–644
Gupta A, Chazen JL, Hartman M et al (2012) Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43:2884–2891
Schoof J, Lubahn W, Baeumer M, Kross R, Wallesch CW, Kozian A, Huth C, Goertler M (2007) Impaired cerebral autoregulation distal to carotid stenosis/occlusion is associated with increased risk of stroke at cardiac surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg 134:690–696
Gonzalez RG, Schaefer PW, Buonanno FS et al (1999) Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 210:155–162
Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M (1993) Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol 142:623–635
Arbelaez A, Castillo M, Mukherji SK (1999) Diffusion-weighted MR imaging of global cerebral anoxia. Am J Neuroradiol 20:999–1007
Rivkin MJ, Volpe JJ (1993) Hypoxic-ischemic brain injury in the newborn. Semin Neurol 13:30–39
Sontheimer H, Kettenmann H, Backus KH, Schachner M (1988) Glutamate opens Na+/K+channels in cultured astrocytes. Glia 1:328–336
Hossmann KA, Hoehn-Berlage M (1995) Diffusion and perfusion MR imaging of cerebral ischemia. Cerebrovasc Brain Metab Rev 7:187–217
Kuhn MJ, Mikulis DJ, Ayoub DM, Kosofsky BE, Davis KR, Taveras JM (1989) Wallerian degeneration after cerebral infarction: evaluation with sequential MR imaging. Radiology 172:179–182
Schaefer PW, Grant PE, Gonzalez RG (2000) Diffusion-weighted MR imaging of the brain. Radiology 217:331–345
Nicholson C, Phillips JM (1981) Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol 321:225–257
Loubinoux I, Volk A, Borredon J, Guirimand S, Tiffon B, Seylaz J, Méric P (1997) Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke 28:419–426
Wu O, Sorensen AG, Benner T, Singhal AB, Furie KL, Greer DM (2009) Comatose patients with cardiac arrest: predicting clinical outcome with diffusion-weighted MR imaging. Radiology 252:173–181
Dani KA, Warach S (2014) Metabolic imaging of ischemic stroke: the present and future. Am J Neuroradiol 35:S37–S43
Hypothermia after Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346:549–556
Nielsen N, Wetterslev J, Cronberg T et al (2013) Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N Engl J Med 369:2197–2206
Callaway CW, Donnino MW, Fink EL et al (2015) Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132:S465–S482
Harada K, Maekawa T, Tsuruta R, Kaneko T, Sadamitsu D, Yamashima T, Yoshida Ki K (2002) Hypothermia inhibits translocation of CaM kinase II and PKC-alpha, beta, gamma isoforms and fodrin proteolysis in rat brain synaptosome during ischemia-reperfusion. J Neurosci Res 67:664–669
Fukuda H, Tomimatsu T, Watanabe N et al (2001) Post-ischemic hypothermia blocks caspase-3 activation in the newborn rat brain after hypoxia-ischemia. Brain Res 910:187–191
Lei B, Adachi N, Arai T (1997) The effect of hypothermia on H2O2 production during ischemia and reperfusion: a microdialysis study in the gerbil hippocampus. Neurosci Lett 222:91–94
Kumar K, Evans AT (1997) Effect of hypothermia on microglial reaction in ischemic brain. Neuroreport 8:947–950
Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T (2001) Intra-ischemic hypothermia attenuates intercellular adhesion molecule-1 (ICAM-1) and migration of neutrophil. Neurol Res 23:105–111
Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA (2002) Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience 114:1081–1090
Gajarski RJ, Smitko K, Despres R, Meden J, Hutton DW (2015) Cost-effectiveness analysis of alternative cooling strategies following cardiac arrest. Springerplus 4:427
Merchant RM, Becker LB, Abella BS, Asch DA, Groeneveld PW (2009) Cost-effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes 2:421–428
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanganalmath, S.K., Gopal, P., Parker, J.R. et al. Global cerebral ischemia due to circulatory arrest: insights into cellular pathophysiology and diagnostic modalities. Mol Cell Biochem 426, 111–127 (2017). https://doi.org/10.1007/s11010-016-2885-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2885-9