Abstract
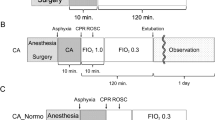
Cardiac arrest (CA) induces whole-body ischemia, causing damage to multiple organs. Ischemic damage to the brain is mainly responsible for patient mortality. However, the molecular mechanism responsible for brain damage is not understood. Prior studies have provided evidence that degradation of membrane phospholipids plays key roles in ischemia/reperfusion injury. The aim of this study is to correlate organ damage to phospholipid alterations following 30 min asphyxia-induced CA or CA followed by cardiopulmonary bypass (CPB) resuscitation using a rat model. Following 30 min CA and CPB resuscitation, rats showed no brain function, moderately compromised heart function, and died within a few hours; typical outcomes of severe CA. However, we did not find any significant change in the content or composition of phospholipids in either tissue following 30 min CA or CA followed by CPB resuscitation. We found a substantial increase in lysophosphatidylinositol in both tissues, and a small increase in lysophosphatidylethanolamine and lysophosphatidylcholine only in brain tissue following CA. CPB resuscitation significantly decreased lysophosphatidylinositol but did not alter the other lyso species. These results indicate that a decrease in phospholipids is not a cause of brain damage in CA or a characteristic of brain ischemia. However, a significant increase in lysophosphatidylcholine and lysophosphatidylethanolamine found only in the brain with more damage suggests that impaired phospholipid metabolism may be correlated with the severity of ischemia in CA. In addition, the unique response of lysophosphatidylinositol suggests that phosphatidylinositol metabolism is highly sensitive to cellular conditions altered by ischemia and resuscitation.





Similar content being viewed by others
References
Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I, Resuscitation Outcomes Consortium I (2008) Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 300:1423–1431. doi:10.1001/jama.300.12.1423
Mongardon N, Dumas F, Ricome S, Grimaldi D, Hissem T, Pene F, Cariou A (2011) Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care 1:45. doi:10.1186/2110-5820-1-45
Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118:2452–2483. doi:10.1161/CIRCULATIONAHA.108.190652
Stub D, Bernard S, Duffy SJ, Kaye DM (2011) Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation 123:1428–1435. doi:10.1161/CIRCULATIONAHA.110.988725
Noseworthy MD, Bray TM (1998) Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radic Biol Med 24:942–951
Friedman J (2011) Why is the nervous system vulnerable to oxidative stress? In: Gadoth N, Göbel HH (eds) Oxidative stress and free radical damage in neurology. Humana Press, Totowa, pp 19–27
Rink C, Khanna S (2011) Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal 14:1889–1903. doi:10.1089/ars.2010.3474
Goto Y, Okamoto S, Yonekawa Y, Taki W, Kikuchi H, Handa H, Kito M (1988) Degradation of phospholipid molecular species during experimental cerebral ischemia in rats. Stroke 19:728–735
Drgova A, Likavcanova K, Dobrota D (2004) Changes of phospholipid composition and superoxide dismutase activity during global brain ischemia and reperfusion in rats. Gen Physiol Biophys 23:337–346
Hamazaki K, Kim HY (2013) Differential modification of the phospholipid profile by transient ischemia in rat hippocampal CA1 and CA3 regions. Prostaglandins Leukot Essent Fat Acids 88:299–306. doi:10.1016/j.plefa.2013.01.003
Rehncrona S, Westerberg E, Akesson B, Siesjo BK (1982) Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem 38:84–93
Moto A, Hirashima Y, Endo S, Takaku A (1991) Changes in lipid metabolites and enzymes in rat brain due to ischemia and recirculation. Mol Chem Neuropathol 14:35–51
Ji J, Baart S, Vikulina AS, Clark RS, Anthonymuthu TS, Tyurin VA, Du L, St Croix CM, Tyurina YY, Lewis J, Skoda EM, Kline AE, Kochanek PM, Wipf P, Kagan VE, Bayir H (2015) Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 35:319–328. doi:10.1038/jcbfm.2014.204
Hossmann KA (1998) Experimental models for the investigation of brain ischemia. Cardiovasc Res 39:106–120
Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R (1995) Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab 15:1032–1039. doi:10.1038/jcbfm.1995.129
Liachenko S, Tang P, Hamilton RL, Xu Y (1998) A reproducible model of circulatory arrest and remote resuscitation in rats for NMR investigation. Stroke 29:1229–1238; discussion 1238–1239
Han F, Boller M, Guo W, Merchant RM, Lampe JW, Smith TM, Becker LB (2010) A rodent model of emergency cardiopulmonary bypass resuscitation with different temperatures after asphyxial cardiac arrest. Resuscitation 81:93–99. doi:10.1016/j.resuscitation.2009.09.018
Niemann JT, Rosborough JP, Walker RG (2004) A model of ischemically induced ventricular fibrillation for comparison of fixed-dose and escalating-dose defibrillation strategies. Acad Emerg Med 11:619–624
Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, Katz L, Ebmeyer E, Safar P (1995) Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 29:249–263
Ballaux PK, Gourlay T, Ratnatunga CP, Taylor KM (1999) A literature review of cardiopulmonary bypass models for rats. Perfusion 14:411–417
White BC, Hildebrandt JF, Evans AT, Aronson L, Indrieri RJ, Hoehner T, Fox L, Huang R, Johns D (1985) Prolonged cardiac arrest and resuscitation in dogs: brain mitochondrial function with different artificial perfusion methods. Ann Emerg Med 14:383–388
Kim J, Yin T, Yin M, Zhang W, Shinozaki K, Selak MA, Pappan KL, Lampe JW, Becker LB (2014) Examination of physiological function and biochemical disorders in a rat model of prolonged asphyxia-induced cardiac arrest followed by cardio pulmonary bypass resuscitation. PLoS One 9:e112012. doi:10.1371/journal.pone.0112012
Kim J, Hoppel CL (2013) Comprehensive approach to the quantitative analysis of mitochondrial phospholipids by HPLC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 912:105–114. doi:10.1016/j.jchromb.2012.10.036
Christiansen K (1975) Lipid extraction procedure for in vitro studies of glyceride synthesis with labeled fatty acids. Anal Biochem 66:93–99
Hsu FF, Turk J (2007) Differentiation of 1-O-alk-1′-enyl-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom 18:2065–2073. doi:10.1016/j.jasms.2007.08.019
Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE (2008) Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem 107:1614–1633. doi:10.1111/j.1471-4159.2008.05728.x
van der Vusse GJ, van Bilsen M, Jans SW, Reneman RS (1996) Lipid metabolism in the ischemic and reperfused heart. EXS 76:175–190
Ruiz Petrich E, Schanne OF, Ponce Zumino A (1996) Electrophysiological responses to ischemia and reperfusion. EXS 76:115–133
van der Vusse GJ, Glatz JF, Stam HC, Reneman RS (1992) Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 72:881–940
Arnsdorf MF, Sawicki GJ (1981) The effects of lysophosphatidylcholine, a toxic metabolite of ischemia, on the components of cardiac excitability in sheep Purkinje fibers. Circ Res 49:16–30
Man RY (1988) Lysophosphatidylcholine-induced arrhythmias and its accumulation in the rat perfused heart. Br J Pharmacol 93:412–416
Friedman J (2011) Why is the nervous system vulnerable to oxidative stress? In: Gadoth N, Göbel HH (eds) Oxidative stress and free radical damage in neurology. Humana Press, Totowa, pp 19–27
Bonventre JV (1999) The 85-kD cytosolic phospholipase A2 knockout mouse: a new tool for physiology and cell biology. J Am Soc Nephrol 10:404–412
Sun D, Gilboe DD (1994) Ischemia-induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J Neurochem 62:1921–1928
Gross RW (1992) Myocardial phospholipases A(2) and their membrane substrates. Trends Cardiovasc Med 2:115–121. doi:10.1016/1050-1738(92)90016-L
Muralikrishna Adibhatla R, Hatcher JF (2006) Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med 40:376–387. doi:10.1016/j.freeradbiomed.2005.08.044
Shanta SR, Choi CS, Lee JH, Shin CY, Kim YJ, Kim KH, Kim KP (2012) Global changes in phospholipids identified by MALDI MS in rats with focal cerebral ischemia. J Lipid Res 53:1823–1831. doi:10.1194/jlr.M022558
Acknowledgment
This work was supported by the NIH Grant (RO1 HL067630).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Human and animal rights and informed consent
This study used animals and the protocol was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. (#803328).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, J., Lampe, J.W., Yin, T. et al. Phospholipid alterations in the brain and heart in a rat model of asphyxia-induced cardiac arrest and cardiopulmonary bypass resuscitation. Mol Cell Biochem 408, 273–281 (2015). https://doi.org/10.1007/s11010-015-2505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2505-0