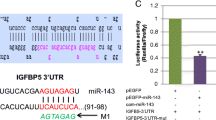
Abstract
MicroRNAs (miRNAs) are short non-coding RNA molecules that perform post-transcriptional repression of target genes by binding to 3′ untranslated regions, and involved in the regulation of many biological processes. Some studies indicate that miRNAs are mechanistically involved in the muscle growth and differentiation. However, little is known about miRNAs expression patterns during the process of bovine skeletal muscle satellite cell myogenic differentiated into myotubes. To investigate the mechanisms of miRNAs-mediated regulation during this process, we performed a miRNAs microarray to detect 783 bovine miRNAs in bovine skeletal muscle satellite cell myogenic differentiation, and the results were further confirmed by a quantitative real-time RT-PCR assay. We observed that the expression of 15 miRNAs was significantly different between bovine skeletal muscle satellite cells and differentiated myotubes, in which twelve were significantly up-regulated and three were down-regulated in myotubes. Furthermore, using bioinformatics methods, the targets of differentially expressed miRNAs were predicted, and were further subjected to gene ontology (GO) and KEGG analysis. A total of 3077 potential target genes were produced, and the highly enriched GOs and KEGG pathways showed that these genes together formed a regulatory network that involved in cell proliferation, cell differentiation, and multiple biological molecular signaling processes. Taken together, the results of the current study suggested the potential regulating roles of these differentially expressed miRNAs in bovine myogenic differentiation.





Similar content being viewed by others
References
Mok GF, Sweetman D (2011) Many routes to the same destination: lessons from skeletal muscle development. Reproduction 141:301–312
Buckingham M (2001) Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 11:440–448
Sokol NS (2012) The role of microRNAs in muscle development. Curr Top Dev Biol 99:59–78
Goljanek-Whysall K, Sweetman D, Münsterberg AE (2012) MicroRNAs in skeletal muscle differentiation and disease. Clin Sci 123(11):611–625
Ge Y, Chen J (2011) MicroRNAs in skeletal myogenesis. Cell Cycle 10(3):441–448
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Grimson A, Farh KKH, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27:91–105
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Hwang HW, Mendell JT (2006) MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 94(6):776–780
Song L, Tuan RS (2006) MicroRNAs and cell differentiation in mammalian development. Birth Defects Res C Embryo Today 78(2):140–149
Guo L, Zhao RC, Wu Y (2011) The role of microRNAs in selfrenewal and differentiation of mesenchymal stem cells. Exp Hematol 39:608–616
Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN (2008) MicroRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 22:3242–3254
Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ (2006) The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38:228–233
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460:705–710
O’Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, Harfe BD (2007) Essential role for Dicer during skeletal muscle development. Dev Biol 311:359–368
Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M (2007) MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 100(3):416–424
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Wang YM, Dai Y, Liu XF, Liu ZW, Li JX, Guo H, Ding XB (2014) Culture, identification and induced differentiation of bovine skeletal muscle satellite cells. China Anim Husb Vet Med 41(7):142–147 (in Chinese)
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 9:185–193
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. PNAS 95:14863–14868
Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, Rasmussen BB (2011) Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics 43:595–603
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Chen JF, Tao YZ, Li J, Deng ZL, Yan Z, Xiao X, Wang DZ (2010) microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol 190:867–879
Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, Conway SJ, Buckingham M (2009) Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. PNAS 106:13383–13387
Dey BK, Gagan J, Dutta A (2011) miR-206 and -486 induce myoblast differentiation by downregulating pax7. Mol Cell Biol 31:203–214
Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D (2008) MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2(3):219–229
Cardinali B, Castellani L, Fasanaro P, Basso A, Alema S, Martelli F, Falcone G (2009) Microrna-221 and microrna-222 modulate differentiation and maturation of skeletal muscle cells. PLoS ONE 4(10):e7607
Shan ZX, Lin QX, Fu YH, Deng CY, Zhou ZL, Zhu JN, Liu XY, Zhang YY, Li Y, Lin SG et al (2009) Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem Biophys Res Commun 381(4):597–601
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI3K/Akt/mTOR and PI3K/Akt/GSK3 pathways. Nat Cell Biol 3(11):1009–1013
Ikeda S, He A, Kong SW, Lu J, Bejar R, Bodyak N, Lee KH, Ma Q, Kang PM, Golub TR, Pu WT (2009) MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol 29:2193–2204
Ren H, Yin P, Duan C (2008) IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J Cell Biol 182(5):979–991
Rosenberg MI, Georges SA, Asawachaicharn A, Analau E, Tapscott SJ (2006) MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J Cell Biol 175:77–85
Chen X, Wang K, Chen J, Guo J, Yin Y, Cai X, Guo X, Wang GQ, Yang R, Zhu LY, Zhang Y, Wang J, Xiang Y, Weng CY, Zen K, Zhang JF, Zhang CY (2009) In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J Biol Chem 284:5362–5369
Sekharam M, Nasir A, Kaiser HE, Coppola D (2003) Insulin-like growth factor 1 receptor activates c-SRC and modifies transformation and motility of colon cancer in vitro. Anticancer Res 23(2B):1517–1524
Sun Q, Wang Y, Zhang Y, Liu F, Cheng X, Hou N, Zhao X, Yang X (2007) Expression profiling reveals dysregulation of cellular cytoskeletal genes in HBx-induced hepatocarcinogenesis. Cancer Biol Ther 6(5):668–674
Lau WM, Weber KL, Doucet M, Chou YT, Brady K, Kowalski J, Tsai HL, Yang J, Kominsky SL (2010) Identification of prospective factors promoting osteotropism in breast cancer: a potential role for CITED2. Int J Cancer 126(4):876–884
Li L, Miyamoto M, Ebihara Y, Mega S, Takahashi R, Hase R, Kaneko H, Kadoya M, Itoh T, Shichinohe T, Hirano S, Kondo S (2006) DRD2/DARPP-32 expression correlates with lymph node metastasis and tumor progression in patients with esophageal squamous cell carcinoma. World J Surg 30(9):1672–1679
Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2838–2849
Schaeffer HJ, Weber MJ (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 19:2435–2444
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252
Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, Kim JK (2008) The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell 133(5):903–915
Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN (2009) MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326:1549–1554
Goljanek-Whysall K, Pais H, Rathjen T, Sweetman D, Dalmay T, Münsterberg A (2012) Regulation of multiple target genes by miR-1 and miR-206 is pivotal for C2C12 myoblast differentiation. J Cell Sci 125:3590–3600
Seale P, Rudnicki MA (2000) A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol 218:115–124
Acknowledgments
We thank Dr. Jianning Liu for his assistance with the bioinformatics analysis. This work was supported by the National Natural Science Foundation of China (31201021), Natural Science Foundation of Tianjin (13JCQNJC14600), and the Excellent Young Teachers Program of Tianjin.
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yi Min Wang and Xiang Bin Ding have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y.M., Ding, X.B., Dai, Y. et al. Identification and bioinformatics analysis of miRNAs involved in bovine skeletal muscle satellite cell myogenic differentiation. Mol Cell Biochem 404, 113–122 (2015). https://doi.org/10.1007/s11010-015-2371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2371-9