Abstract
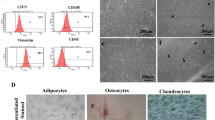
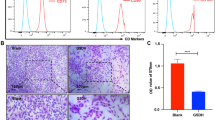
This study was designed to investigate the effect and mechanism of lipopolysaccharide (LPS) preconditioning on survival and connexin 43 (CX43) expression in rat bone marrow mesenchymal stem cells (bMSCs) under hypoxia and serum deprivation (Hypoxia/SD) conditions. Whole marrow cells were obtained from the femora and tibiae of SD rats, and bMSCs were isolated by density gradient centrifugation and attachment culture. Surface antigens were determined by FACS before the experiment using antibodies conjugated directly against anti-rat CD34, anti-CD45, anti-CD29, and anti-CD44. Passage 3 bMSCs were used for all experiments. The effect of LPS preconditioning on bMSCs apoptosis in response to Hypoxia/SD was investigated by an Annexin V-FITC/PI binding assay and a mitochondrial membrane potential (△Ψm) assay. Cyc-c released into the cytosol from mitochondria and CX43 in bMSCs was determined by Western blot before and after LPS preconditioning. Subsequently, extracellular signal-regulated kinase (ERK) was inhibited with PD98059 to analyze the role of ERK in modulating CX43 expression after LPS preconditioning. The bMSCs surface antigen profiles obtained by flow cytometry were positive for CD29 and CD44 and negative for CD34 and CD45. The Hypoxia/SD conditions induced significant apoptosis of bMSCs. Compared with the Hypoxia/SD group, cells treated with LPS prevented △Ψm from falling significantly. LPS inhibited Hypoxia/SD-induced Cyc-c release. These results were consistent with the total analysis of apoptosis of MSCs. Compared with the control group, the level of CX43 expression in the Hypoxia/SD group and LPS + Hypoxia/SD group decreased significantly at each time point. The level of CX43 expression in the Hypoxia/SD group was lower than that in the LPS + Hypoxia/SD group, while the difference was not significant between the PD98059 + LPS + Hypoxia/SD group and the PD98059 + Hypoxia/SD group (P > 0.05). Compared with the LPS + Hypoxia/SD group, CX43 level in the PD98059 + LPS + Hypoxia/SD group and PD98059 + Hypoxia/SD group decreased significantly (P < 0.05). These results demonstrated that Hypoxia/SD conditions could induce apoptosis of bMSCs markedly. Low-dose LPS preconditioning may preserve the mitochondrial function by maintaining the mitochondrial transmembrane potential and inhibiting Cyc-c release in Hypoxia/SD-induced bMSCs apoptosis. LPS preconditioning also had a stabilizing effect on the cell membrane by inhibiting the decrease of CX43, and this modulating mechanism may be related to the ERK signaling pathway.







Similar content being viewed by others
References
Kemp CD, Conte JV (2012) The pathophysiology of heart failure. Cardiovasc Pathol 21(5):365–371
Zhen H, Wang J, Xue L et al (2012) LPS-pretreated bone marrow stem cells as potential treatment for myocardial infarction. Front Biosci 17:1294–1303
Trachtenberg B, Velazquez DL, Williams AR et al (2011) Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J 161(3):487–493
Brenner C, Franz WM (2011) The use of stem cells for the repair of cardiac tissue in ischemic heart disease. Expert Rev Med Devices 8(2):209–225
Wang JA, Chen TL, Jiang J et al (2004) Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 29(1):74–82
Suzuki Y, Kim HW, Ashraf M et al (2010) Diazoxide potentiates mesenchymal stem cell survival via NF-κB-dependent miR-146a expression by targeting Fas. Am J Physiol Heart Circ Physiol 299(4):H1077–H1082
Wang ZJ, Zhang FM, Wang LS et al (2009) Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3 K/Akt. Cell Biol Int 33(6):665–674
Xu R, Chen J, Cong X et al (2008) Lovastatin protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3 K/Akt and ERK1/2. J Cell Biochem 103(1):256–269
Schulz R, Boengler K, Totzeck A et al (2007) Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev 12(3–4):261–266
Snykers S, Vanhaecke T, Rogiers V (2006) Isolation of Rat Bone Marrow Stem Cells. Methods Mol Biol 320:265–272
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105(1):93–98
Müller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH (2006) Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol 41(5):876–884
Lee R, Pulin A, Seo M et al (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5:54–63
Zangi L, Margalit R, Reich-Zeliger S et al (2009) Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 27(11):2865–2874
Yao Y, Zhang F, Wang L, Zhang G, Wang Z, Chen J, Gao X (2009) Lipopolysaccharide preconditioning enhances the efficacy of mesenchymal stem cells transplantation in a rat model of acute myocardial infarction. J Biomed Sci 16:74
Zhu W, Chen J, Cong X, Hu S, Chen X (2006) Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 24(2):416–425
Beardslee MA, Lerner DL, Tadros PN et al (2000) Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 87:656–662
Jain SK, Schuessler RB, Saffitz JE (2003) Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ Res 92:1138–1144
Srisakuldee W, Jeyaraman MM, Nickel BE, Tanguy S, Jiang ZS, Kardami E (2009) Phosphorylation of connexin-43 at serine 262 promotes a cardiac injury-resistant state. Cardiovasc Res 83:672–681
Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J (2008) Nedergaard M.A central role of connexin 43 in hypoxic preconditioning. J Neurosci 28(3):681–695
Berthoud VM, Minogue PJ, Laing JG, Beyer EC (2004) Pathways for degradation of connexins and gap junctions. Cardiovasc Res 62(2):256–267
VanSlyke JK, Musil LS (2005) Cytosolic stress reduces degradation of connexin 43 internalized from the cell surface and enhances gap junction formation and function. Mol Biol Cell 16(11):5247–5257
Jia G, Cheng G, Gangahar DM, Agrawal DK (2008) Involvement of connexin 43 in angiotensin II-induced migration and proliferation of saphenous vein smooth muscle cells via the MAPK-AP-1 signaling pathway. J Mol Cell Cardiol 44(5):882–890
Cushing P, Bhalla R, Johnson AM, Rushlow WJ, Meakin SO, Belliveau DJ (2005) Nerve growth factor increases connexin 43 phosphorylation and gap junctional intercellular communication. J Neurosci Res 82(6):788–801
Boswell BA, Le AC, Musil LS (2009) Upregulation and maintenance of gap junctional communication in lens cells. Exp Eye Res 88(5):919–927
Acknowledgments
The authors thank all the individuals who voluntarily participated in the study and Prof. Xueying Xie (the Research Center of Learning Science, Southeast University, Nanjing, China) for her insightful suggestions on the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Li, Z., Zhang, Y. et al. CX43 change in LPS preconditioning against apoptosis of mesenchymal stem cells induced by hypoxia and serum deprivation is associated with ERK signaling pathway. Mol Cell Biochem 380, 267–275 (2013). https://doi.org/10.1007/s11010-013-1683-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1683-x