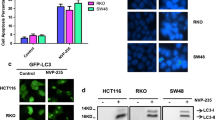
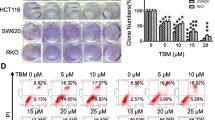
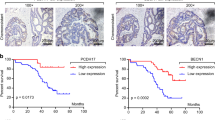
Abstract
Colorectal cancer is the second leading cause of cancer-related deaths. Drug resistance and/or off-target toxicity against normal cells limit the effectiveness of current chemotherapies for the treatment of colorectal cancer. In the current study, we studied the potential cytotoxic effects of short-chain and cell-permeable C6 ceramide in cultured colorectal cancer HT-29 cells and focused on the underlying mechanisms. We observed that C6 ceramide-induced HT-29 cell death and growth inhibition in a dose- and time-dependent manner. However, no significant apoptosis was observed in C6 ceramide-treated HT-29 cells. Our data support that autophagy contributed to C6 ceramide-induced cytotoxic effects, as autophagy inhibitors, 3-methyladenine (3-MA) and hydroxychloroquine, inhibited C6 ceramide’s effect; however, autophagy activators, everolimus (RAD001) and temsirolimus, mimicked C6 ceramide effects and induced HT-29 cell death. Further, we indentified that AMP-activated protein kinase (AMPK)/Ulk1 signaling was required for autophagy induction by C6 ceramide, and AMPK silencing by a specific short hairpin RNA suppressed C6 ceramide-induced autophagy and cytotoxic effects. Reversely, forced activation of AMPK by its activator AICAR or by genetic manipulation caused autophagic death in HT-29 cells, which was inhibited by 3-MA. Our results suggest that autophagy, but not apoptosis, is a major contributor for C6 ceramide-induced cytotoxic effects in HT-29 cells, and activation of AMPK/Ulk1 is required for the process.





Similar content being viewed by others
Abbreviations
- 3-MA:
-
3-Methyladenine
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- AICAR:
-
5-Aminoimidazole-4-carboxamide ribotide
- JNK:
-
c-Jun N-terminal kinase
- LC3B:
-
Light chain 3B
- MTT:
-
3-[4,5-Dimethylthylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- mTOR:
-
Mammalian target or rapamycin
- mTORC1:
-
mTOR complex 1
- sh-RNA:
-
Short hairpin RNA
- PI:
-
Propidium iodide
References
Chen A, Xu J, Johnson AC (2006) Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 25:278–287
Mohandas KM, Desai DC (1999) Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol 18:118–121
Gustin DM, Brenner DE (2002) Chemoprevention of colon cancer: current status and future prospects. Cancer Metastasis Rev 21:323–348
Gorlick R, Bertino JR (1999) Drug resistance in colon cancer. Semin Oncol 26:606–611
Longley DB, Allen WL, Johnston PG (2006) Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta 1766:184–196
Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D (2012) Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 367:1616–1625
Lemos C, Sack U, Schmid F, Juneja M, Stein U (2012) Anti-metastatic treatment in colorectal cancer: targeting signaling pathways. Curr Pharm Des 19:841–863
Fillet M, Bentires-Alj M, Deregowski V, Greimers R, Gielen J, Piette J, Bours V, Merville MP (2003) Mechanisms involved in exogenous C2- and C6-ceramide-induced cancer cell toxicity. Biochem Pharmacol 65:1633–1642
Mao C, Obeid LM (2008) Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta 1781:424–434
Lin CF, Chen CL, Lin YS (2006) Ceramide in apoptotic signaling and anticancer therapy. Curr Med Chem 13:1609–1616
Dimanche-Boitrel MT, Rebillard A, Gulbins E (2011) Ceramide in chemotherapy of tumors. Recent Pat Anticancer Drug Discov 6:284–293
Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS (2008) Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood 111:4365–4374
Barak A, Morse LS, Goldkorn T (2001) Ceramide: a potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42:247–254
Flowers M, Fabrias G, Delgado A, Casas J, Abad JL, Cabot MC (2012) C6-ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res Treat 133:447–458
Kurinna SM, Tsao CC, Nica AF, Jiffar T, Ruvolo PP (2004) Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res 64:7852–7856
Hartfield PJ, Mayne GC, Murray AW (1997) Ceramide induces apoptosis in PC12 cells. FEBS Lett 401:148–152
Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, Salas A, Ogretmen B (2010) Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol 6:1603–1624
Martin D, Salinas M, Fujita N, Tsuruo T, Cuadrado A (2002) Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem 277:42943–42952
Ji C, Yang B, Yang YL, He SH, Miao DS, He L, Bi ZG (2010) Exogenous cell-permeable C6 ceramide sensitizes multiple cancer cell lines to doxorubicin-induced apoptosis by promoting AMPK activation and mTORC1 inhibition. Oncogene 29:6557–6568
Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC (2005) Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene 24:178–187
Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH, Ai X (2012) Ursolic acid-induced AMP-activated protein kinase (AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cells. Biochem Biophys Res Commun 419:714–747
Abreu-Martin MT, Vidrich A, Lynch DH, Targan SR (1995) Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-alpha and ligation of Fas antigen. J Immunol 155:4147–4154
Vaculova A, Hofmanova J, Soucek K, Kovarikova M, Kozubik A (2002) Tumor necrosis factor-alpha induces apoptosis associated with poly(ADP-ribose) polymerase cleavage in HT-29 colon cancer cells. Anticancer Res 22:1635–1639
Craighead MW, Tiwari P, Keynes RG, Waters CM (1999) Human oligodendroglial cell line, MO3.13, can be protected from apoptosis using the general caspase inhibitor zVAD-FMK. J Neurosci Res 57:236–243
Seglen PO, Gordon PB (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA 79:1889–1892
Amaravadi RK, Thompson CB (2007) The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res 13:7271–7279
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101:3329–3335
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13:2004–2008
Chen MB, Shen WX, Yang Y, Wu XY, Gu JH, Lu PH (2011) Activation of AMP-activated protein kinase is involved in vincristine-induced cell apoptosis in B16 melanoma cell. J Cell Physiol 226:1915–1925
Chen MB, Wu XY, Gu JH, Guo QT, Shen WX, Lu PH (2010) Activation of AMP-activated protein kinase contributes to doxorubicin-induced cell death and apoptosis in cultured myocardial H9c2 cells. Cell Biochem Biophys 60:311–322
Rocha GZ, Dias MM, Ropelle ER, Osorio-Costa F, Rossato FA, Vercesi AE, Saad MJ, Carvalheira JB (2011) Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res 17:3993–4005
Sun H, Yu T, Li J (2011) Co-administration of perifosine with paclitaxel synergistically induces apoptosis in ovarian cancer cells: more than just AKT inhibition. Cancer Lett 310:118–128
Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, Yang Y (2010) Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem 285:40461–40471
Chen MB, Wu XY, Gu JH, Guo QT, Shen WX, Lu PH (2011) Activation of AMP-activated protein kinase contributes to doxorubicin-induced cell death and apoptosis in cultured myocardial H9c2 cells. Cell Biochem Biophys 60:311–322
Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH, Ai X (2012) Ursolic acid-induced AMP-activated protein kinase (AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cells. Biochem Biophys Res Commun 419:741–747
Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D (2012) Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Res 14:R35
Kang MR, Park SK, Lee CW, Cho IJ, Jo YN, Yang JW, Kim JA, Yun J, Lee KH, Kwon HJ, Kim BW, Lee K, Kang JS, Kim HM (2012) Widdrol induces apoptosis via activation of AMP-activated protein kinase in colon cancer cells. Oncol Rep 27:1407–1412
Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ (2007) Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett 247:115–121
Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ (2010) Induction of apoptosis by quercetin is mediated through AMPKalpha1/ASK1/p38 pathway. Cancer Lett 292:228–236
Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS, Lim JS (2012) Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem Pharmacol 83:385–394
Jang KY, Jeong SJ, Kim SH, Jung JH, Kim JH, Koh W, Chen CY, Kim SH (2012) Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett 319:197–202
Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, Van de Casteele M (2003) AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol 30:151–161
Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L (2002) Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett 526:38–42
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18:283–293
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Carling D, Sanders MJ, Woods A (2008) The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 32(Suppl 4):S55–S59
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2:21–33
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19
Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL (2010) ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci USA 107:4153–4158
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688
Gozuacik D, Kimchi A (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23:2891–2906
Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12(Suppl 2):1509–1518
Rosenfeldt MT, Ryan KM (2011) The multiple roles of autophagy in cancer. Carcinogenesis 32:955–963
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968
Hardie DG (2008) AMPK and Raptor: matching cell growth to energy supply. Mol Cell 30:263–265
Lee JW, Park S, Takahashi Y, Wang HG (2010) The association of AMPK with ULK1 regulates autophagy. PLoS One 5:e15394
Shang L, Chen S, Du F, Li S, Zhao L, Wang X (2011) Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci USA 108:4788–4793
Hardie DG, Ross FA, Hawley SA (2012) AMP-Activated Protein Kinase: a target for drugs both ancient and modern. Chem Biol 19:1222–1236
Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG (2012) Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 142(1504–1515):e1503
Acknowledgments
Funding/Support: This study was supported in part by the National Natural Science Foundation of China.
Conflict of interests
The authors have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huo, Hz., Wang, B., Qin, J. et al. AMP-activated protein kinase (AMPK)/Ulk1-dependent autophagic pathway contributes to C6 ceramide-induced cytotoxic effects in cultured colorectal cancer HT-29 cells. Mol Cell Biochem 378, 171–181 (2013). https://doi.org/10.1007/s11010-013-1608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1608-8