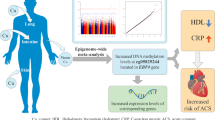
Abstract
The association between oxidative stress and coronary artery disease (CAD) is well documented. However, the role of epigenetic factors contributing to oxidative stress is relatively unexplored. In this study, we aimed to explore the impact of DNA methylation profile in BCL2/E1B adenovirus interacting protein 3 (BNIP3), extracellular superoxide dismutase (EC-SOD) and glutathione-S-transferase P1 (GSTP1) on the oxidative stress in CAD. Further, the contribution of folate pathway genetic polymorphisms in regulating epigenome was elucidated. The expression of BNIP3, EC-SOD, and GSTP1 were studied by using Maxima@SYBR-green based real-time qPCR approach in peripheral blood samples. Combined bisulfite restriction analysis and methylation-specific PCR were used to study promoter CpG island methylation. Further, the effect of homocysteine on BNIP3 gene expression was studied in human aortic endothelial cells in vitro. CAD cases exhibited upregulation of BNIP3, downregulation of EC-SOD and GSTP1. Hypomethylation of BNIP3 and hypermethylation of EC-SOD were observed in CAD cases. The expression of BNIP3 was positively correlated with homocysteine, MDA, protein carbonyls, and methylene tetrahydrofolate reductase C677T, while showing inverse association with cytosolic serine hydroxymethyl transferase C1420T. The expressions of EC-SOD and GSTP1 showed positive association with thymidylate synthase (TYMS) 2R3R, while inverse association with MDA, protein carbonyls, and methionine synthase reductase (MTRR) A66G. In vitro analysis showed homocysteine-dependent upregulation of BNIP3. The results of this study suggest that the aberrations in one-carbon metabolism appear to induce altered gene expression of EC-SOD, GSTP1, and BNIP3, and thus contribute to the increased oxidative stress and increased susceptibility to CAD.




Similar content being viewed by others
Abbreviations
- CAD:
-
Coronary artery disease
- BNIP3:
-
Bcl2/adenovirus E1B interacting protein-3
- EC-SOD:
-
Extracellular superoxide dismutase
- GSTP1:
-
Glutathione-S-transferase pi
- 8-OxodG:
-
8-Oxo-2′-deoxyguanosine
- MDA:
-
Malondialdehyde
- HAECs:
-
Human aortic endothelial cells
- DEPC:
-
Diethylpyrocarbonate
- COBRA:
-
Combined bisulfite restriction analysis
- MSP:
-
Methylation-specific PCR
- ROS:
-
Reactive oxygen species
- EBM:
-
Endothelial basal medium
- FBS:
-
Foetal bovine serum
- GCPII C1561T:
-
Glutamate carboxypeptidase II
- MTHFR C677T:
-
Methylene tetrahydrofolate homocysteine methyltransferase
- MTRR A66G:
-
Methionine synthase reductase
- cSHMT C1420T:
-
Cytosolic serine hydroxymethyl transferase
- TYMS:
-
Thymidylate synthase 5′-UTR 28 bp tandem repeat
References
Turunen MP, Aavik E, Ylä-Herttuala S (2009) Epigenetics and atherosclerosis. Biochim Biophys Acta 1790(9):886–891
Guay SP, Brisson D, Munger J, Lamarche B, Gaudet D, Bouchard L (2012) ABCA1 gene promoter DNA methylation is associated with HDL particle profile and coronary artery disease in familial hypercholesterolemia. Epigenetics 7(5):464–472
Roberts R, Stewart AF (2012) The genetics of coronary artery disease. Curr Opin Cardiol 27(3):221–227
Ghassibe-Sabbagh M, Platt DE, Youhanna S, Abchee AB, Stewart K, Badro DA, Haber M, Salloum AK, Douaihy B, El Bayeh H, Othman R, Shasha N, Kibbani S, Chammas E, Milane A, Nemr R, Kamatani Y, Hager J, Cazier JB, Gauguier D, Zalloua PA, FGENTCARD Consortium (2012) Genetic and environmental influences on total plasma homocysteine and its role in coronary artery disease risk. Atherosclerosis 222(1):180–186
Tayal D, Goswami B, Tyagi S, Chaudhary M, Mallika V (2012) Interaction between dyslipidemia, oxidative stress and inflammatory response in patients with angiographically proven coronary artery disease. Cardiovasc J Afr 23(1):23–27
Rajesh KG, Surekha RH, Mrudula SK, Prasad Y, Sanjib KS, Prathiba N (2011) Oxidative and nitrosative stress in association with DNA damage in coronary heart disease. Singap Med J 52(4):283–288
Nagayoshi Y, Kawano H, Hokamaki J, Uemura T, Soejima H, Kaikita K, Sugiyama S, Yamabe H, Shioji I, Sasaki S, Kuroda Y, Ogawa H (2009) Differences in oxidative stress markers based on the aetiology of heart failure: comparison of oxidative stress in patients with and without coronary artery disease. Free Radic Res 43(12):1159–1166
Park E, Kyoung Park Y, Kim SM, Lee HJ, Kang MH (2009) Susceptibility to oxidative stress is greater in Korean patients with coronary artery disease than healthy subjects. J Clin Biochem Nutr 45(3):341–346
Vijaya Lakshmi SV, Naushad SM, Rupasree Y, Seshagiri Rao D, Kutala VK (2011) Interactions of 5′-UTR thymidylate synthase polymorphism with 677C → T methylene tetrahydrofolate reductase and 66A → G methyltetrahydrofolate homocysteine methyl-transferase reductase polymorphisms determine susceptibility to coronary artery disease. J Atheroscler Thromb 18(1):56–64
Vijaya Lakshmi SV, Naushad SM, Seshagiri Rao D, Kutala VK (2011) Oxidative stress is associated with genetic polymorphisms in one-carbon metabolism in coronary artery disease. Cell Biochem Biophys. doi:10.1007/s12013-011-9322-1
Divyya S, Naushad SM, Addlagatta A, Murthy PV, Reddy ChR, Digumarti RR, Gottumukkala SR, Kumar A, Rammurti S, Kutala VK (2012) Paradoxical role of C1561T glutamate carboxypeptidase II (GCPII) genetic polymorphism in altering disease susceptibility. Gene 497(2):273–279
Naushad SM, Pavani A, Digumarti RR, Gottumukkala SR, Kutala VK (2011) Epistatic interactions between loci of one-carbon metabolism modulate susceptibility to breast cancer. Mol Biol Rep 38(8):4893–4901
Govindaiah V, Naushad SM, Prabhakara K, Krishna PC, Radha Rama Devi A (2009) Association of parental hyperhomocysteinemia and C677T methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy loss. Clin Biochem 42(4–5):380–386
Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G → T and A → C substitutions. J Biol Chem 267(1):166–172
Welch GN, Upchurch GR Jr, Loscalzo J (1997) Homocysteine, oxidative stress, and vascular disease. Hosp Pract 32(6):81–92
Siow YL, Au-Yeung KK, Woo CW, O K (2006) Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J 398(1):73–82
Alvarez-Maqueda M, El Bekay R, Monteseirín J, Alba G, Chacón P, Vega A, Santa María C, Tejedo JR, Martín-Nieto J, Bedoya FJ, Pintado E, Sobrino F (2004) Homocysteine enhances superoxide anion release and NADPH oxidase assembly by human neutrophils. Effects on MAPK activation and neutrophil migration. Atherosclerosis 172(2):229–238
Lin HC, Song TY, Hu ML (2011) S-Adenosylhomocysteine enhances DNA damage through increased β-amyloid formation and inhibition of the DNA-repair enzyme OGG1b in microglial BV-2 cells. Toxicology 290(2–3):342–349
Bagnyukova TV, Powell CL, Pavliv O, Tryndyak VP, Pogribny IP (2008) Induction of oxidative stress and DNA damage in rat brain by a folate/methyl-deficient diet. Brain Res 1237:44–51
Naushad SM, Reddy CA, Rupasree Y, Pavani A, Digumarti RR, Gottumukkala SR, Kuppusamy P, Kutala VK (2011) Cross-talk between one-carbon metabolism and xenobiotic metabolism: implications on oxidative DNA damage and susceptibility to breast cancer. Cell Biochem Biophys 61(3):715–723
Pogribny IP, James SJ, Beland FA (2012) Molecular alterations in hepatocarcinogenesis induced by dietary methyl deficiency. Mol Nutr Food Res 56(1):116–125
Majumdar S, Mukherjee S, Maiti A, Karmakar S, Das AS, Mukherjee M, Nanda A, Mitra C (2009) Folic acid or combination of folic acid and vitamin B(12) prevents short-term arsenic trioxide-induced systemic and mitochondrial dysfunction and DNA damage. Environ Toxicol 24(4):377–387
Naushad SM, Prayaga A, Digumarti RR, Gottumukkala SR, Kutala VK (2012) Bcl-2/adenovirus E1B 19 kDa-interacting protein 3 (BNIP3) expression is epigenetically regulated by one-carbon metabolism in invasive duct cell carcinoma of breast. Mol Cell Biochem 361(1–2):189–195
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 97(16):9082–9087
Su XD, Li SS, Tian YQ, Zhang ZY, Zhang GZ, Wang LX (2011) Elevated serum levels of advanced glycation end products and their monocyte receptors in patients with type 2 diabetes. Arch Med Res 42(7):596–601
Nihei S, Tasaki H, Yamashita K, Ozumi K, Morishita T, Tsutsui M, Okazaki M, Nakashima Y, Adachi T (2004) Hyperhomocysteinemia is associated with human coronary atherosclerosis through the reduction of the ratio of endothelium-bound to basal extracellular superoxide dismutase. Circ J 68(9):822–828
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
van Diest PJ, Suijkerbuijk KP, Koop EA, de Weger RA, van der Wall E (2010) Low levels of BNIP3 promoter hypermethylation in invasive breast cancer. Anal Cell Pathol 33(3):175–176
Murai M, Toyota M, Satoh A, Suzuki H, Akino K, Mita H, Sasaki Y, Ishida T, Shen L, Garcia-Manero G, Issa JP, Hinoda Y, Tokino T, Imai K (2005) Aberrant DNA methylation associated with silencing BNIP3 gene expression in haematopoietic tumours. Br J Cancer 92(6):1165–1172
Murai M, Toyota M, Suzuki H, Satoh A, Sasaki Y, Akino K, Ueno M, Takahashi F, Kusano M, Mita H, Yanagihara K, Endo T, Hinoda Y, Tokino T, Imai K (2005) Aberrant methylation and silencing of the BNIP3 gene in colorectal and gastric cancer. Clin Cancer Res 11(3):1021–1027
Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB (2008) Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 295(5):H2025-31. Epub 2008 Sep 12
Oikawa S, Hirosawa I, Hirakawa K, Kawanishi S (2001) Site specificity and mechanism of oxidative DNA damage induced by carcinogenic catechol. Carcinogenesis 22(8):1239–1245
Mohammad NS, Yedluri R, Addepalli P, Gottumukkala SR, Digumarti RR, Kutala VK (2011) Aberrations in one-carbon metabolism induce oxidative DNA damage in sporadic breast cancer. Mol Cell Biochem 349(1–2):159–167
Ramprasath T, Senthil Murugan P, Prabakaran AD, Gomathi P, Rathinavel A, Selvam GS (2011) Potential risk modifications of GSTT1, GSTM1 and GSTP1 (glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochem Biophys Res Commun 407(1):49–53
Nomani H, Mozafari H, Ghobadloo SM, Rahimi Z, Raygani AV, Rahimi MA, Haghi AF, Keshavarz AA (2011) The association between GSTT1, M1, and P1 polymorphisms with coronary artery disease in Western Iran. Mol Cell Biochem 354(1–2):181–187
Zhang J, Ye J, Altafaj A, Cardona M, Bahi N, Llovera M, Cañas X, Cook SA, Comella JX, Sanchis D (2011) EndoG links Bnip3-induced mitochondrial damage and caspase-independent DNA fragmentation in ischemic cardiomyocytes. PLoS ONE 6(3):e17998
Lee SJ, Kim KM, Namkoong S, Kim CK, Kang YC, Lee H, Ha KS, Han JA, Chung HT, Kwon YG, Kim YM (2005) Nitric oxide inhibition of homocysteine-induced human endothelial cell apoptosis by down-regulation of p53-dependent Noxa expression through the formation of S-nitrosohomocysteine. J Biol Chem 280(7):5781–5788
Acknowledgments
We acknowledge Council of Scientific and Industrial Research (CSIR), New Delhi for providing Senior Research Fellowship (SRF) to SVV. VKK and KS are recipients of Ramanujan Fellowship awarded by Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshmi, S.V.V., Naushad, S.M., Reddy, C.A. et al. Oxidative stress in coronary artery disease: epigenetic perspective. Mol Cell Biochem 374, 203–211 (2013). https://doi.org/10.1007/s11010-012-1520-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1520-7