Abstract
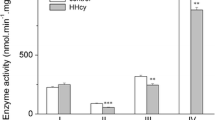
Tissue accumulation of homocysteine occurs in classical homocystinuria, a metabolic disease characterized biochemically by cystathionine β-synthase deficiency. Vascular manifestations such as myocardial infarction, cerebral thrombosis, hepatic steatosis, and pulmonary embolism are common in this disease and poorly understood. In this study, we investigated the effect of chronic hyperhomocysteinemia on some parameters of oxidative stress (thiobarbituric acid-reactive substances, protein carbonyl content, 2′,7′-dichlorofluorescein fluorescence assay, and total radical-trapping antioxidant potent) and activities of antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) in the rat lung. Reduced glutathione content and glucose 6-phosphate dehydrogenase activity, as well as nitrite levels, were also evaluated. Wistar rats received daily subcutaneous injections of Hcy (0.3–0.6 μmol/g body weight) from the 6th to the 28th days-of-age and the control group received saline. One and 12 h after the last injection, rats were killed and the lungs collected. Hyperhomocysteinemia increased lipid peroxidation and oxidative damage to protein, and disrupted antioxidant defenses (enzymatic and non-enzymatic) in the lung of rats, characterizing a reliable oxidative stress. In contrast, this amino acid did not alter nitrite levels. Our findings showed a consistent profile of oxidative stress in the lung of rats, elicited by homocysteine, which could explain, at least in part, the mechanisms involved in the lung damage that is present in some homocystinuric patients.






Similar content being viewed by others
References
Diaz-Arrastia R (2000) Homocysteine and neurologic disease. Arch Neurol 57:1422–1427
Andersson A, Ankerst J, Lindgren A, Larsson K, Hultberg B (2001) Hyperhomocysteinemia and changed plasma thiol redox status in chronic obstructive pulmonary disease. Clin Chem Lab Med 39:229–233
Mattson MP, Kruman II, Duan W (2002) Folic acid and homocysteine in age-related disease. Ageing Res Rev 1:95–111
Bottiglieri T (2005) Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry 29:1103–1112
Obeid R, McCaddon A, Herrmann W (2007) The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med 45:1590–1606
Herrmann W, Lorenzl S, Obeid R (2007) Review of the role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric disorders—current evidence and preliminary recommendations. Fortschr Neurol Psychiatr 75:515–527
Mudd SH, Levy HL, Skovby F (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 1279–1327
Klastin G, Conn HO (1993) Hepatic lesions in disorders of protein and amino acid metabolism. Oxford University Press, New York
van der Berg M, van der Knapp MS, Boers GH, Stehouwer CD, Rauwerda JA, Valk J (1995) Hyperhomocysteinemia; with reference to its neuroradiological aspects. Neuroradiology 37:403–411
Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050
Kim WK, Pae YS (1996) Involvement of N-methyl-d-aspartate receptor and free radical in homocysteine-mediated toxicity on rat cerebellar granule cells in culture. Neurosci Lett 216:117–120
Zhang F, Slungaard A, Vercellotti GM, Ladecola C (1998) Superoxide-dependent cerebrovascular effects of homocysteine. Am J Physiol 274:R1704–R1711
Ho PI, Ashline D, Dhitavat S, Ortiz D, Collins SC, Shea TB, Rogers E (2003) Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol Dis 14:32–42
Jara-Prado A, Ortega-Vazquez A, Martinez-Ruano L, Rios C, Santamaria A (2003) Homocysteine-induced brain lipid peroxidation: effects of NMDA receptor blockade, antioxidant treatment, and nitric oxide synthase inhibition. Neurotox Res 5:237–243
Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR (2004) Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 35:1957–1962
Faraci FM, Lentz SR (2004) Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke 35:345–347
Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC (2005) Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289:H2649–H2656
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G (2007) Vascular oxidative stress in Alzheimer disease. J Neurol Sci 257:240–246
Shi H, Liu KJ (2007) Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci 12:1318–1328
Muhl D (2008) Changes in oxidative stress and hemostatic parameters in the course of thrombolytic therapy of pulmonary embolism. Orv Hetil 149:935–948
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York, pp 79–185
Wyse AT, Zugno AI, Streck EL, Matté C, Calcagnotto T, Wannmacher CM, Wajner M (2002) Inhibition of Na+, K+-ATPase activity in hippocampus of rats subjected to acute administration of homocysteine is prevented by vitamins E and C treatment. Neurochem Res 27:1685–1689
Streck EL, Vieira PS, Wannmacher CM, Dutra-Filho C, Wajner M, Wyse AT (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18:147–154
Matté C, Monteiro SC, Calcagnotto T, Bavaresco CS, Netto CA, Wyse AT (2004) In vivo and in vitro effects of homocysteine on Na+, K+-ATPase activity in parietal, prefrontal and cingulate cortex of young rats. Int J Dev Neurosci 22:185–190
Matté C, Scherer EB, Stefanello FM, Barschak AG, Vargas CR, Netto CA, Wyse AT (2007) Concurrent folate treatment prevents Na+, K+-ATPase activity inhibition and memory impairments caused by chronic hyperhomocysteinemia during rat development. Int J Dev Neurosci 25:545–552
Matté C, Stefanello FM, Mackedanz V, Pederzolli CD, Lamers ML, Dutra-Filho CS, dos Santos MF, Wyse AT (2009) Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci 27:337–344
Streck EL, Matté C, Vieira PS, Rombaldi F, Wannmacher CM, Wajner M, Wyse AT (2002) Reduction of Na+, K+-ATPase activity in hippocampus of rats subjected to chemically induced hyperhomocysteinemia. Neurochem Res 27:1593–1598
Llesuy SF, Milei J, Molina H, Boveris A, Milei S (1985) Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4′-epiadriamycin in mice. Tumori 71:241–249
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Stadtman ER (1990) Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9:315–325
Stadtman ER, Levine RL (2003) Free-radical mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
LeBel CP, Ali SF, McKee M, Bondy SC (1990) Organometal-induced increases in oxygen reactive species: the potential of 2′,7′-dichlorofluorescein diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol 104:17–24
Lissi E, Pascual C, Del Castillo MD (1992) Luminol luminescence induced by 2,2′-azo-bis-(2-amidinopropane) thermolysis. Free Radic Res Commun 17:299–311
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388:261–266
Marklund SL (1985) Pyrogallol autoxidation. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 243–247
Aebi H (1984) Catalase, in vitro. Methods Enzymol 105:121–126
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352
Leong SF, Clark JB (1984) Regional enzyme development in rat brain. Enzymes associated with glucose utilization. Biochem J 218:131–138
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Clarke R, Daly L, Robinson K (1999) Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 324:1149–1155
Mattson MP, Haberman F (2003) Folate and homocysteine metabolism: therapeutic targets in cardiovascular and neurodegenerative disorders. Curr Med Chem 10:1923–1929
Jiang H, Wang XF, Fang L, Tang C, Zhu Y, Wang X (2005) Upregulation of aldose reductase by homocysteine in type II alveolar epithelial cells. Biochem Biophys Res Commun 337:1084–1091
Hamelet J, Maurin N, Fulchiron R, Delabar JM, Janel N (2007) Mice lacking cystathionine beta synthase have lung fibrosis and air space enlargement. Exp Mol Pathol 83:249–253
Dikshit M, Srivastava R, Srimal RC (1989) Role of free radicals in pulmonary thromboembolism in mice. Thromb Res 55:549–557
Ryrfeldt A, Bannenberg G, Moldeus P (1993) Free radicals and lung disease. Br Med Bull 49:588–603
Jacobsen DW (1998) Homocysteine and vitamins in cardiovascular disease. Clin Chem 44:1833–1843
Loscalzo J (1996) The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 98:5–7
Mansoor MA, Bergmark C, Svardal AM, Lonning PE, Ueland PM (1995) Redox status and protein binding of plasma homocyst(e)ine and other aminothiols in patients with early-onset peripheral vascular disease. Arterioscler Thromb Vasc Biol 15:232–240
Matté C, Mackedanz V, Stefanello FM, Scherer EB, Andreazza AC, Zanotto C, Moro AM, Garcia SC, Gonçalves CA, Erdtmann B, Salvador M, Wyse AT (2009) Chronic hyperhomocysteinemia alters antioxidant defenses and increases DNA damage in brain and blood os rats: protective effect of folic acid. Neurochem Int 54:7–13
Grisham MB, McCord J (1986) Chemistry and cytotoxicity of reactive oxygen metabolites. In: Taylor AE, Matalon S, Ward P (eds) Physiology of oxygen radicals. Williams and Wilkins Co, Baltimore, pp 1–18
Alvarez-Maqueda M, El Bekay R, Monteseirín J, Alba G, Chacón P, Vega A, Santa María C, Tejedo JR, Martín-Nieto J, Bedoya FJ, Pintado E, Sobrino F (2004) Homocysteine enhances superoxide anion release and NADPH oxidase assembly by human neutrophils. Effects on MAPK activation and neutrophil migration. Atherosclerosis 172:229–238
Fridorich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247:1–11
Blum J, Fridorich I (1985) Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys 240:500–508
Vessey DA, Lee KH (1993) Inactivation of enzymes of the glutathione antioxidant system by treatment of culture human keratinocytes with peroxides. J Invest Dermatol 100:829–833
Heinecke JW, Rosen H, SuzukiI LA, Chait A (1987) The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein in arterial smooth muscle cells. J Biol Chem 21:10098–10103
Upchurch GR Jr, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF Jr, Loscalzo J (1997) Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 272:17012–17017
Stern F, Berner YN, Polyak Z, Komarnitsky M, Sela BA, Hopp M, Dror Y (2004) Homocysteine effect on protein degradation rates. Clin Biochem 37:1002–1009
Sharma P, Senthilkumar RD, Brahmachari V, Sundaramoorthy E, Mahajan A, Sharma A, Sengupta S (2006) Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis 5:1–19
Kletzien RF, Harris PK, Foellmi LA (1994) Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J 8:174–181
Hashida K, Sakakura Y, Makino N (2002) Kinetic studies on the hydrogen peroxide elimination by cultured PC12 cells: rate limitation by glucose-6-phosphate dehydrogenase. Biochim Biophys Acta 1572:85–90
Rakhit RD, Mojet MH, Marber MS, Duchen MR (2001) Mitochondria as targets for nitric oxide-induced protection during simulated ischemia and reoxygenation in isolated neonatal cardiomyocytes. Circulation 103:2617–2623
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxinitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271:C1424–C1437
Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE (1995) Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr 62:S1490–S1500
Acknowledgments
This work was supported in part by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, RS, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Cunha, A.A., Ferreira, A.G.K., da Cunha, M.J. et al. Chronic hyperhomocysteinemia induces oxidative damage in the rat lung. Mol Cell Biochem 358, 153–160 (2011). https://doi.org/10.1007/s11010-011-0930-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0930-2