Abstract
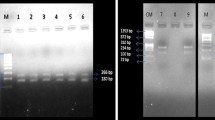
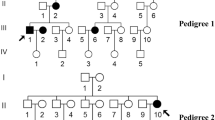
Involvement of mitochondrial and nuclear gene mutations in the development of type 2 diabetes (T2D) has been established well in various populations around the world. Previously, we have found the mitochondrial A>G transition at nucleotide position 3243 and 8296 in the T2D patients of Coimbatore population. This study is aimed to screen for the presence of various mitochondrial and nuclear DNA mutations in the T2D patients of Coimbatore to identify most prevalent mutation. This helps in identifying the susceptible individuals based on their clinical phenotype in future. Blood samples were collected from 150 unrelated late-onset T2D patients and 100 age-matched unrelated control samples according to World Health Organization criteria. Genotyping for the selected genes was done by polymerase chain reaction–single strand confirmation polymorphism, direct sequencing, and polymerase chain reaction–restriction fragment length polymorphism. The mitochondrial T>C transition at 8356 and nuclear-encoded GLUT1 gene mutation were found in the selected T2D patients. The T8356C mutation was found in two patients (1.3%), and the clinical characteristics were found to be similar in both the patients whereas GLUT1 gene mutation was found in seven patients. Four out of seven patients showed homozygous (−) genotype and three patients showed heterozygous (±) genotype for the mutant allele XbaI. Among these three patients, one patient was found to have elevated level of urea and creatinine with the history of kidney dysfunction and chronic T2D. Our results suggest that the T8356C and GLUT1 gene mutations may have an important role in developing late-onset T2D in Coimbatore population. Particularly, individuals with GLUT1 gene may develop kidney dysfunction at their later age.



Similar content being viewed by others
References
King H, Aubert RE, Herman WH (1998) Global burden of diabetes. Prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
Yajnik CS (1998) The insulin resistance epidemic in India: small at birth, big as adult?. IDF Bull 43:23–28
Ahuja MMS (ed) (1979) Epidemiological studies on diabetes mellitus in India. In: Epidemiology of diabetes in developing countries. Interprint, New Delhi, pp 29–38
Ramachandran C, Snehalatha E, Latha et al (1997) Rising prevalence of NIDDM in an urban population in India. Diabetologia 40:232–237
Arora MM, Chander Y, Rai R (2000) Diabetes mellitus in India-Y2K not ok. Med J Armed Forces India 56:1–2
Florentz C (2002) Molecular investigations on tRNAs involved in human mitochondrial disorders. Biosci Rep 22(1):81–98
Yu P, Yu D, Liu D, Wang K, Tang X (2004) Relationship between mutations of mitochondrial DNA ND1 gene and type 2 diabetes. Chin Med J 117(7):985–989
Liu SM, Zhou X, Zheng F, Li X, Liu F, Zhang HM, Xie Yan (2007) Novel mutations found in mitochondrial diabetes in Chinese Han population. Diabetes Res Clin Pract 76(3):425–435
Ming-zhen L, De-min Y, Pei Y, De-min L, Kun W, Xin-zhi T (2008) Mitochondrial gene mutations and type 2 diabetes in Chinese families. Chin Med J 121(8):682–686
Deng GH, Zhou X, Pang ZY, Liu SM, Xie Y (2009) Study on mitochondrial function of ND1 gene with 3316 G–>A mutation in human diabetes. Zhonghua Yi Xue Za Zhi 89(40):2822–2826
Liu ZH, Guan TJ, Chen ZH et al (1999) Glucose transporter (GLUT1) allele (XbaI−) associated with nephropathy in non-insulin-dependent diabetes mellitus. Kidney Int 55:1843–1848
World Health Organization (1999) Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1. Diagnosis and classification of diabetes mellitus. World Health Organization, Geneva
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3):1215
van den Bosch BJC, de Coo RFM, Scholte HR, Nijland JG, van den Ruud B, de Visser M, de Die-Smulders CEM, Smeets HJM (2000) Mutation analysis of the entire mitochondrial genome using denaturing high performance liquid chromatography. Nucleic Acids Res 28(20):e89
Hodgkinson AD, Millward BA, Demaine AG (2001) Polymorphisms of the glucose transporter (GLUT1) gene are associated with diabetic nephropathy. Kidney Int 59:985–989
Craig SS, Kevin RS, Maureen LB, Jill MS, Sreekumaran KN (2003) Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001
Orita M, Suzuki Y, Sekiya T, Hayashi K (1989) Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics 5:874–879
Bataillard M, Chatzoglou E, Rumbach L, Sternberg D, Tournade A, Laforet P, Jardel C, Maisonobe T, Lombes A (2001) Atypical MELAS syndrome associated with a new mitochondrial tRNA glutamine point mutation. Neurology 56:405–407
Uusimaa J, Finnila S, Remes AM, Rantala H, Vainionnaa L, Hassinin IE, Majamaa K (2004) Molecular epimdemiology of childhood mitochondrial encephalomyopathies in a Finnish population: Sequence analysis of entire mtDNA of 17 children reveals heteroplasmic mutations in tRNAArg, tRNAGlu and tRNALeu (UUR) genes. Pediatrics 114(2):443–450
Duraisamy P, Santhini E, Vijaya Padma V, Balamurugan R (2010) Prevalence of mitochondrial tRNA gene mutations and their association with specific clinical phenotypes in type-ii diabetes mellitus patients of Coimbatore. Genet Test Mol Biomarkers 14(1):49–55
Thorand B, Liese AD, Metzger MH, Reitmeir P, Schneider A, Lowel H (2001) Can inaccuracy of reported parental history of diabetes explain the maternal transmission hypothesis for diabetes? Int J Epidemiol 30:1084–1089
Van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA et al (1992) Mutation in mitochondrial tRNA Leu(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 5:368–371
Silvestri G, Moraes CT, Shanske S, Oh SJ, DiMauro S (1992) A new mtDNA mutation in the tRNA(Lys) gene associated with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet 51:1213–1217
Heilig C, Zaloga C, Lee M, Zhao X, Riser B, Brosius F, Cortes P (1995) Immuno gold localization of high-affinity glucose transporter isoform in normal rat kidney. Lab Invest 73:674–684
Heilig CW, Liu Y, England RL, Freytag SO, Gilbert JD, Heilig KO, Zhu M, Concepcion LA, Brosius FC III (1997) d-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-1 sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes 46:1030–1039
Parving HH, Osterby R, Anderson PW, Hsueh WA (1996) Diabetic nephropathy. In: Brenner BM (ed) The kidney. WB Saunders Company, Philadelphia, pp 1864–1891
Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani S, Deepa R, Mohan V (2007) Prevalence and risk factors of diabetic nephropathy in an urban south Indian population. The Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 30:2019–2024
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11010-010-0676-2
Rights and permissions
About this article
Cite this article
Vijaya Padma, V., Anitha, S., Santhini, E. et al. Mitochondrial and nuclear gene mutations in the type 2 diabetes patients of Coimbatore population. Mol Cell Biochem 345, 223–229 (2010). https://doi.org/10.1007/s11010-010-0576-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0576-5