Abstract
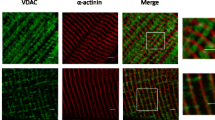
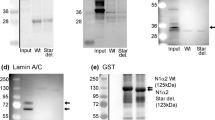
We studied possible connections of tubulin, microtubular system, and microtubular network stabilizing STOP protein with mitochondria in rat and mouse cardiac and skeletal muscles by confocal microscopy and oxygraphy. Intracellular localization and content of tubulin was found to be muscle type-specific, with high amounts in oxidative muscles, and much lower in glycolytic skeletal muscle. STOP protein localization and content in muscle cells was also muscle type-specific. In isolated heart mitochondria, addition of 1 μM tubulin heterodimer increased apparent K m for ADP significantly. Dissociation of microtubular system into free tubulin by colchicine treatment only slightly decreased initially high apparent K m for ADP in permeabilized cells, and diffusely distributed free tubulin stayed inside the cells, obviously connected to the intracellular structures. To identify the genes that are specific for oxidative muscle, we developed and applied a method of kindred DNA. The results of sequencing and bioinformatic analysis of isolated cDNA pool common for heart and m. soleus showed that in adult mice the β-tubulin gene is expressed predominantly in oxidative muscle cells. It is concluded that whereas dimeric tubulin may play a significant role in regulation of mitochondrial outer membrane permeability in the cells in vivo, its organization into microtubular network has a minor significance on that process.









Similar content being viewed by others
Abbreviations
- STOP:
-
Stabilizing tubule only polypeptide
- CK:
-
Creatine kinase
- MtCK:
-
Mitochondrial creatine kinase
- ANT:
-
Adenine nucleotide translocase
- VDAC:
-
Voltage-dependant anion channel
- DTT:
-
Dithiothreitol
References
Vendelin M, Béraud N, Guerrero K, Andrienko T et al (2005) Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol 288:C757–C767
Saks V, Kuznetsov A, Andrienko T, Usson Y et al (2003) Heterogeneity of ADP diffusion and regulation of respiration in cardiac cells. Biophys J 84:3436–3456
Kuznetsov AV, Tiivel T, Sikk P, Kaambre T et al (1996) Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem 241:909–915
Voloshchuk SG, Belikova YO, Klyushnik TP, Benevolensky DS (1998) Comparative study of respiration kinetics and protein composition of skinned fibers from various types of rat muscle. Biochemistry 63:155–158
Saks VA, Kaambre T, Sikk P, Eimre M et al (2001) Intracellular energetic units in red muscle cells. Biochem J 356:643–657
Seppet EK, Kaambre T, Sikk P, Tiivel T et al (2001) Functional complexes of mitochondria with Ca, MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta 1504:379–395
Appaix F, Kuznetsov AV, Usson Y, Kay L et al (2003) Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Exp Physiol 88:175–190
Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C et al (2008) Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci USA 105(48):18746–18751
Guzun R, Timohhina N, Tepp K, Monge C et al (2009) Regulation of respiration controlled by mitochondrial creatine kinase in permeabilized cardiac cells in situ. Importance of system level properties. Biochim Biophys Acta 1787:1089–1105
Tagawa H, Koide M, Sato H, Zile MR et al (1998) Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ Res 82:751–761
Pabion M, Job D, Margolis RL (1984) Sliding of STOP proteins on microtubules. Biochemistry 23:6642–6648
Baratier J, Peris L, Brocard J, Gory-Fauré S et al (2006) Phosphorylation of microtubule-associated protein STOP by calmodulin kinase II. J Biol Chem 281(28):19561–19569
Makarov AA, Tsvetkov PO, Villard C, Esquieu D (2007) Vinflunine, a novel microtubule inhibitor, suppresses calmodulin interaction with the microtubule-associated protein STOP. Biochemistry 46(51):14899–14906
Aguezzoul M, Andrieux A, Denarier E (2003) Overlap of promoter and coding sequences in the mouse STOP gene (Mtap6). Genomics 81:623–627
Andrieux A, Salin PA, Vernet M et al (2002) The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioural disorders. Genes Dev 16:2350–2364
Fradley RL, O’Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS (2005) STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res 163(2):257–264
Kay L, Li Z, Mericskay M, Olivares J et al (1997) Study of regulation of mitochondrial respiration in vivo. An analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochim Biophys Acta 1322:41–59
Saks VA, Chernousova GB, Gukovsky DE, Smirnov VN (1975) Studies of energy transport in heart cells. Mitochondrial isoenzyme of creatine phosphokinase: kinetic properties and regulatory action of Mg2+ ions. Eur J Biochem 57:273–290
Booth RF, Clark JB (1978) A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J 176:365–370
Saks VA, Veksler VI, Kuznetsov AV, Kay L et al (1998) Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184:81–100
Kuznetsov AV, Veksler V, Gellerich FN, Saks V et al (2008) Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3(6):965–976
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold spring Harbor laboratory, Cold Spring Harbor, NewYork
Chenchik AY, Zhu L, Diachenko R, Li J et al (1989) Generation and use of high-quality cDNA from small amounts of RNA by SMART PCR. In Siebert PD, Larrick JW (eds) Gene cloning and analysis by RT-PCR. BioTechniques Books, Natick, pp 305–3194
Puurand U, Kadaja L, Seppet EK (2003) Kindred DNA amplification from two distinct populations of cDNA fragments. Biotechniques 34:994–1000
Ogata T, Yamasaki Y (1997) Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248:214–223
Gueguen N, Lefaucheur L, Fillaut M, Vincent A (2005) Control of skeletal muscle mitochondria respiration by adenine nucleotides: differential effect of ADP and ATP according to muscle contractile type in pigs. Comp Biochem Physiol B 140:287–297
Birkedal R, Gesser H (2004) Regulation of mitochondrial energy production in cardiac cells of rainbow trout (Oncorhynchus mykiss). J Comp Physiol B 174:255–262
Seppet EK, Eimre M, Andrienko T, Kaambre T et al (2004) Studies of mitochondrial respiration in muscle cells in situ: use and misuse of experimental evidence in mathematical modelling. Mol Cell Biochem 256–257:219–227
Andrienko T, Kuznetsov AV, Kaambre T, Usson Y et al (2003) Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. J Exp Biol 206:2059–2072
Monge C, Beraud N, Kuznetsov AV, Rostovtseva T et al (2008) Regulation of respiration in brain mitochondria and synaptosomes: restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol Cell Biochem 318(1–2):147–165
Altschul SF, Madden TL, Schäffer AA, Zhang J et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Luedeke JD, McCall RD, Dillaman RM, Kinsey ST (2004) Properties of slow- and fast-twitch skeletal muscle from mice with an inherited capacity for hypoxic exercise. Comp Biochem Physiol 138:373–382
Mänttäri S, Järvilehto M (2005) Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC Physiol 5(1):4
Linden M, Karlsson G (1996) Identification of porin as a binding site for MAP2. Biochem Biophys Res Commun 218:833–836
Lewis SA, Lee MG, Gowan NJ (1985) Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol 101:852–861
Carre M, Andre N, Carles G, Borghi H et al (2002) Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem 277:33664–33669
Acknowledgements
The authors would like to acknowledge Annie Schweitzer, Dominique Proietto, and Jose Olivares (LBFA, Grenoble, France) for skillful technical assistance, and Didier Job and Annie Andrieux (Grenoble Neurosciences Institute, La Tronche, France) for useful discussion. We are grateful to Dan L. Sackett (Laboratory of Integrative and Medical Biophysics) and Tatiana K. Rostovtseva (Laboratory of Physical and Structural Biology), NICHD, NIH, Bethesda, USA, for supplying us the purified heterodimer of tubulin. This work was supported by INSERM, France, by Agence National de la Recherche (contract ANR-07-BLAN-0086, project R0711CC), France, to C.M. and V.S., by grants of Estonian Science Foundation (No 7117 and 7823).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerrero, K., Monge, C., Brückner, A. et al. Study of possible interactions of tubulin, microtubular network, and STOP protein with mitochondria in muscle cells. Mol Cell Biochem 337, 239–249 (2010). https://doi.org/10.1007/s11010-009-0304-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0304-1