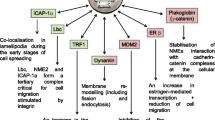
Abstract
Nm23-H1 (also known as NDPKA) and h-Prune form a protein complex that is part of a little-understood protein network. Modifications of this complex correlate with cancer status. Here, we focus on the role of the Nm23-H1–h-Prune complex in cellular physiology, through an analysis of the balance between the ‘bound’ and ‘non-bound’ states of Nm23-H1 and h-Prune, whereby we speculate on the ‘read-out’ during cell homeostasis under non-balanced conditions. We have analysed the biochemical activities of both Nm23-H1 and h-Prune alone and in combination, focussing on the anti-metastatic activity of Nm23-H1. We have then investigated the cellular mechanisms responsible for the formation of the Nm23-H1–h-Prune complex. To evaluate the importance of the equilibrium between the formation of the Nm23-H1–h-Prune complex and the ‘free’ levels of Nm23-H1 and h-Prune, we propose a model based on a pro-cancer condition where this equilibrium is negatively affected.

Similar content being viewed by others
References
Steeg PS, Bevilacqua G, Pozzatti R, Liotta LA, Sobel ME (1988) Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Res 48:6550–6554
Reymond A, Volorio S, Merla G, Al-Maghtheh M, Zuffardi O, Bulfone A, Ballabio A, Zollo M (1999) Evidence for interaction between human PRUNE and nm23-H1 NDPKinase. Oncogene 18:7244–7252. doi:10.1038/sj.onc.1203140
Mochizuki T, Bilitou A, Waters CT, Hussain K, Zollo M, Ohnuma SI (2009) Xenopus NM23-X4 regulates retinal gliogenesis through interaction with p27Xic1. Neural Dev 4:1. doi:10.1186/1749-8104-4-1
Tseng YH, Vicent D, Zhu J, Niu Y, Adeyinka A, Moyers JS, Watson PH, Kahn CR (2001) Regulation of growth and tumorigenicity of breast cancer cells by the low molecular weight GTPase Rad and nm23. Cancer Res 61:2071–2079
Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, Kahn CR (1999) Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci USA 96:14911–14918. doi:10.1073/pnas.96.26.14911
Otsuki Y, Tanaka M, Yoshii S, Kawazoe N, Nakaya K, Sugimura H (2001) Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc Natl Acad Sci USA 98:4385–4390. doi:10.1073/pnas.071411598
Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C (2002) ARF6-GTP recruits Nm23–H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 4:929–936. doi:10.1038/ncb881
Subramanian C, Cotter MAII, Robertson ES (2001) Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23-H1: a molecular link to cancer metastasis. Nat Med 7:350–355. doi:10.1038/85499
Hartsough MT, Morrison DK, Salerno M, Palmieri D, Ouatas T, Mair M, Patrick J, Steeg PS (2002) Nm23-H1 metastasis suppressor phosphorylation of kinase suppressor of Ras via a histidine protein kinase pathway. J Biol Chem 277:32389–32399. doi:10.1074/jbc.M203115200
D’Angelo A, Garzia L, Andre A, Carotenuto P, Aglio V, Guardiola O, Arrigoni G, Cossu A, Palmieri G, Aravind L, Zollo M (2004) Prune cAMP phosphodiesterase binds nm23-H1 and promotes cancer metastasis. Cancer Cell 5:137–149. doi:10.1016/S1535-6108(04)00021-2
Timmons L, Shearn A (1996) Germline transformation using a prune cDNA rescues prune/killer of prune lethality and the prune eye color phenotype in Drosophila. Genetics 144:1589–1600
Garzia L, D’Angelo A, Amoresano A, Knauer SK, Cirulli C, Campanella C, Stauber RH, Steegborn C, Iolascon A, Zollo M (2008) Phosphorylation of nm23-H1 by CKI induces its complex formation with h-prune and promotes cell motility. Oncogene 27:1853–1864. doi:10.1038/sj.onc.1210822
Middelhaufe S, Garzia L, Ohndorf UM, Kachholz B, Zollo M, Steegborn C (2007) Domain mapping on the human metastasis regulator protein h-Prune reveals a C-terminal dimerization domain. Biochem J 407:199–205. doi:10.1042/BJ20070408
Zollo M, Andre A, Cossu A, Sini MC, D’Angelo A, Marino N, Budroni M, Tanda F, Arrigoni G, Palmieri G (2005) Overexpression of h-prune in breast cancer is correlated with advanced disease status. Clin Cancer Res 11:199–205
Marino N, Zollo M (2007) Understanding h-prune biology in the fight against cancer. Clin Exp Metastasis 24:637–645. doi:10.1007/s10585-007-9109-3
Tammenkoski M, Koivula K, Cusanelli E, Zollo M, Steegborn C, Baykov AA, Lahti R (2008) Human metastasis regulator protein H-prune is a short-chain exopolyphosphatase. Biochemistry 47:9707–9713. doi:10.1021/bi8010847
Beavo JA, Brunton LL (2002) Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol 3:710–718. doi:10.1038/nrm911
Lugnier C (2006) Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109:366–398. doi:10.1016/j.pharmthera.2005.07.003
Meinkoth JL, Alberts AS, Went W, Fantozzi D, Taylor SS, Hagiwara M, Montminy M, Feramisco JR (1993) Signal transduction through the cAMP-dependent protein kinase. Mol Cell Biochem 127–128:179–186. doi:10.1007/BF01076769
D’Angelo A, Zollo M (2004) Unraveling genes and pathways influenced by H-prune PDE overexpression: a model to study cellular motility. Cell Cycle 3:758–761
Kulaev IS, Kulakovskaya TV, Andreeva NA, Lichko LP (1999) Metabolism and function of polyphosphates in bacteria and yeast. Prog Mol Subcell Biol 23:27–43
Lichko LP, Andreeva NA, Kulakovskaya TV, Kulaev IS (2003) Exopolyphosphatases of the yeast Saccharomyces cerevisiae. FEMS Yeast Res 3:233–238. doi:10.1016/S1567-1356(02)00205-2
Garzia L, Roma C, Tata N, Pagnozzi D, Pucci P, Zollo M (2006) H-prune-nm23-H1 protein complex and correlation to pathways in cancer metastasis. J Bioenerg Biomembr 38:205–213. doi:10.1007/s10863-006-9036-z
Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR (1992) Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta 1114:147–162
Woodgett JR (1991) A common denominator linking glycogen metabolism, nuclear oncogenes and development. Trends Biochem Sci 16:177–181. doi:10.1016/0968-0004(91)90071-3
Kobayashi T, Hino S, Oue N, Asahara T, Zollo M, Yasui W, Kikuchi A (2006) Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions. Mol Cell Biol 26:898–911. doi:10.1128/MCB.26.3.898-911.2006
Schafer DA, Cooper JA (1995) Control of actin assembly at filament ends. Annu Rev Cell Dev Biol 11:497–518. doi:10.1146/annurev.cb.11.110195.002433
Ouatas T, Salerno M, Palmieri D, Steeg PS (2003) Basic and translational advances in cancer metastasis: Nm23. J Bioenerg Biomembr 35:73–79. doi:10.1023/A:1023497924277
Hartsough MT, Steeg PS (2000) Nm23/nucleoside diphosphate kinase in human cancers. J Bioenerg Biomembr 32:301–308. doi:10.1023/A:1005597231776
Postel EH (1998) NM23-NDP kinase. Int J Biochem Cell Biol 30:1291–1295. doi:10.1016/S1357-2725(98)00087-9
Boissan M, Poupon MF, Lacombe ML (2007). NM23 and metastasis suppressor genes: update. Med Sci (Paris) 23:1115–1123
Salerno M, Ouatas T, Palmieri D, Steeg PS (2003) Inhibition of signal transduction by the nm23 metastasis suppressor: possible mechanisms. Clin Exp Metastasis 20:3–10. doi:10.1023/A:1022578000022
Ma D, McCorkle JR, Kaetzel DM (2004) The metastasis suppressor NM23–H1 possesses 3′-5′ exonuclease activity. J Biol Chem 279:18073–18084. doi:10.1074/jbc.M400185200
Kaetzel DM, Zhang Q, Yang M, McCorkle JR, Ma D, Craven RJ (2006) Potential roles of 3′-5′ exonuclease activity of NM23-H1 in DNA repair and malignant progression. J Bioenerg Biomembr 38:163–167. doi:10.1007/s10863-006-9040-3
Kimura N, Shimada N, Ishijima Y, Fukuda M, Takagi Y, Ishikawa N (2003) Nucleoside diphosphate kinases in mammalian signal transduction systems: recent development and perspective. J Bioenerg Biomembr 35:41–47. doi:10.1023/A:1023489722460
Dorsam RT, Gutkind JS (2007) G-protein-coupled receptors and cancer. Nat Rev Cancer 7:79–94. doi:10.1038/nrc2069
Ohkura N, Kishi M, Tsukada T, Yamaguchi K (2001) Menin, a gene product responsible for multiple endocrine neoplasia type 1, interacts with the putative tumor metastasis suppressor nm23. Biochem Biophys Res Commun 282:1206–1210. doi:10.1006/bbrc.2001.4723
Symons M, Settleman J (2000) Rho family GTPases: more than simple switches. Trends Cell Biol 10:415–419. doi:10.1016/S0962-8924(00)01832-8
Freije JM, Blay P, MacDonald NJ, Manrow RE, Steeg PS (1997) Site-directed mutation of Nm23–H1. Mutations lacking motility suppressive capacity upon transfection are deficient in histidine-dependent protein phosphotransferase pathways in vitro. J Biol Chem 272:5525–5532. doi:10.1074/jbc.272.9.5525
Zhou Q, Yang X, Zhu D, Ma L, Zhu W, Sun Z, Yang Q (2007) Double mutant P96S/S120G of Nm23-H1 abrogates its NDPK activity and motility-suppressive ability. Biochem Biophys Res Commun 356:348–353. doi:10.1016/j.bbrc.2007.02.066
Forus A, D’Angelo A, Henriksen J, Merla G, Maelandsmo GM, Florenes VA, Olivieri S, Bjerkehagen B, Meza-Zepeda LA, del Vecchio Blanco F, Muller C, Sanvito F, Kononen J, Nesland JM, Fodstad O, Reymond A, Kallioniemi OP, Arrigoni G, Ballabio A, Myklebost O, Zollo M (2001) Amplification and overexpression of PRUNE in human sarcomas and breast carcinomas—a possible mechanism for altering the nm23-H1 activity. Oncogene 20:6881–6890. doi:10.1038/sj.onc.1204874
Oue N, Yoshida K, Noguchi T, Sentani K, Kikuchi A, Yasui W (2007) Increased expression of h-prune is associated with tumor progression and poor survival in gastric cancer. Cancer Sci 98:1198–1205. doi:10.1111/j.1349-7006.2007.00515.x
Noguchi T, Oue N, Wada S, Sentani K, Sakamoto N, Kikuchi A, Yasui W (2009) h-prune is an independent prognostic marker for survival in esophageal squamous cell carcinoma. Ann Surg Oncol 16:1390–1396. doi:10.1245/s10434-007-9585-3
Postel EH (2003) Multiple biochemical activities of NM23/NDP kinase in gene regulation. J Bioenerg Biomembr 35:31–40. doi:10.1023/A:1023485505621
Bevilacqua G, Sobel ME, Liotta LA, Steeg PS (1989) Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Res 49:5185–5190
Florenes VA, Aamdal S, Myklebost O, Maelandsmo GM, Bruland OS, Fodstad O (1992) Levels of nm23 messenger RNA in metastatic malignant melanomas: inverse correlation to disease progression. Cancer Res 52:6088–6091
Yamaguchi A, Urano T, Goi T, Takeuchi K, Niimoto S, Nakagawara G, Furukawa K, Shiku H (1994) Expression of human nm23-H1 and nm23-H2 proteins in hepatocellular carcinoma. Cancer 73:2280–2284. doi:10.1002/1097-0142(19940501)73:9<2280::AID-CNCR2820730908>3.0.CO;2-3
Malins DC, Polissar NL, Gunselman SJ (1996) Tumor progression to the metastatic state involves structural modifications in DNA markedly different from those associated with primary tumor formation. Proc Natl Acad Sci USA 93:14047–14052. doi:10.1073/pnas.93.24.14047
Haut M, Steeg PS, Willson JK, Markowitz SD (1991) Induction of nm23 gene expression in human colonic neoplasms and equal expression in colon tumors of high and low metastatic potential. J Natl Cancer Inst 83:712–716. doi:10.1093/jnci/83.10.712
Muller W, Schneiders A, Hommel G, Gabbert HE (1998) Expression of nm23 in gastric carcinoma: association with tumor progression and poor prognosis. Cancer 83:2481–2487. doi:10.1002/(SICI)1097-0142(19981215)83:12<2481::AID-CNCR11>3.0.CO;2-P
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW (1997) Gene expression profiles in normal and cancer cells. Science 276:1268–1272. doi:10.1126/science.276.5316.1268
Carotenuto P, Marino N, Bello AM, D’Angelo A, Di Porzio U, Lombardi D, Zollo M (2006) PRUNE and NM23-M1 expression in embryonic and adult mouse brain. J Bioenerg Biomembr 38:233–246. doi:10.1007/s10863-006-9044-z
Gervasi F, D’Agnano I, Vossio S, Zupi G, Sacchi A, Lombardi D (1996) nm23 influences proliferation and differentiation of PC12 cells in response to nerve growth factor. Cell Growth Differ 7:1689–1695
Dabernat S, Larou M, Masse K, Dobremez E, Landry M, Mathieu C, Daniel JY (1999) Organization and expression of mouse nm23–M1 gene. Comparison with nm23-M2 expression. Gene 236:221–230. doi:10.1016/S0378-1119(99)00288-7
Masse K, Dabernat S, Bourbon PM, Larou M, Amrein L, Barraud P, Perel Y, Camara M, Landry M, Lacombe ML, Daniel JY (2002) Characterization of the nm23-M2, nm23-M3 and nm23-M4 mouse genes: comparison with their human orthologs. Gene 296:87–97. doi:10.1016/S0378-1119(02)00836-3
Arnaud-Dabernat S, Bourbon PM, Dierich A, Le Meur M, Daniel JY (2003) Knockout mice as model systems for studying nm23/NDP kinase gene functions. Application to the nm23-M1 gene. J Bioenerg Biomembr 35:19–30. doi:10.1023/A:1023561821551
Srivastava S, Zhdanova O, Di L, Li Z, Albaqumi M, Wulff H, Skolnik EY (2008) Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3.1. Proc Natl Acad Sci USA 105:14442–14446. doi:10.1073/pnas.0803678105
Cuello F, Schulze RA, Heemeyer F, Meyer HE, Lutz S, Jakobs KH, Niroomand F, Wieland T (2003) Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gbeta subunits. Complex formation of NDPK B with Gbeta gamma dimers and phosphorylation of His-266 IN Gbeta. J Biol Chem 278:7220–7226. doi:10.1074/jbc.M210304200
Ljubimov AV, Caballero S, Aoki AM, Pinna LA, Grant MB, Castellon R (2004) Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci 45:4583–4591. doi:10.1167/iovs.04-0686
Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC (1998) p53 deficiency and misexpression of protein kinase CK2alpha collaborate in the development of thymic lymphomas in mice. Oncogene 16:2965–2974. doi:10.1038/sj.onc.1201854
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC (2001) Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 20:3247–3257. doi:10.1038/sj.onc.1204411
Engel M, Issinger OG, Lascu I, Seib T, Dooley S, Zang KD, Welter C (1994) Phosphorylation of nm23/nucleoside diphosphate kinase by casein kinase 2 in vitro. Biochem Biophys Res Commun 199:1041–1048. doi:10.1006/bbrc.1994.1334
Han KY, Hong BS, Yoon YJ, Yoon CM, Kim YK, Kwon YG, Gho YS (2007) Polyphosphate blocks tumour metastasis via anti-angiogenic activity. Biochem J 406:49–55. doi:10.1042/BJ20061542
Acknowledgements
This work was supported by grants from the FP6-E.E.T pipeline LSH-CT-2006-037260, TuMIC LSH-CT HEALTH-2007-2.4.1-6 and Associazione Italiana per la Lotta al Neuroblastoma, an Associazione Italiana per la Lotta al Neuroblastoma Research Fellowship (A.G.) and an AIRC 2007-8 grant. We also thank the CEINGE services laboratory for several of the strategies that were developed for the use of proteomic and translational approaches within these projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galasso, A., Zollo, M. The Nm23-H1–h-Prune complex in cellular physiology: a ‘tip of the iceberg’ protein network perspective. Mol Cell Biochem 329, 149–159 (2009). https://doi.org/10.1007/s11010-009-0115-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0115-4