Abstract
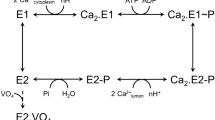
We identified α2, α1, and β1 isoforms of Na+/K+-ATPase in caveolae vesicles of bovine pulmonary smooth muscle plasma membrane. The biochemical and biophysical characteristics of the α2β1 isozyme of Na+/K+-ATPase from caveolae vesicles were studied during solubilization and purification using the detergents 1,2-heptanoyl-sn-phosphatidylcholine (DHPC), poly(oxy-ethylene)8-lauryl ether (C12E8), and Triton X-100, and reconstitution with the phospholipid dioleoyl-phosphatidylcholine (DOPC). DHPC was determined to be superior to C12E8, whereas C12E8 was better than Triton X-100 in the active enzyme yields and specific activity. Fluorescence studies with DHPC-purified α2β1 isozyme of Na+/K+-ATPase elicited higher E1Na−E2 K transition compared with that of the C12E8- and Triton X-100-purified enzyme. The rate of Na+ efflux in DHPC–DOPC-reconstituted isozyme was higher compared to the C12E8–DOPC- and Triton X100–DOPC-reconstituted enzyme. Circular dichroism analysis suggests that the DHPC-purified α2β1 isozyme of Na+/K+-ATPase possessed more organized secondary structure compared to the C12E8- and Triton X-100-purified isozyme.









Similar content being viewed by others
References
Jorgensen PL (1982) Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+-K+)-ATPase. Biochim Biophys Acta 694:27–68
Skou JC, Esmann M (1992) The Na, K-ATPase. J Bioenerg Biomembr 24:249–261
Pressley TA (1996) Structure and function of the Na, K pump: ten years of molecular biology. Miner Electrolyte Metab 22:264–271
Jorgensen PL, Nielsen JM, Rasmussen JH, Pedersen PA (1998) Structure-function relationships based on ATP binding and cation occlusion at equilibrium in Na+/K+-ATPase. Acta Physiol Scand Suppl 643:79–87
Orlowski J, Lingrel JB (1988) Tissue-specific and developmental regulation of rat Na, K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem 263:10436–10442
Juhaszova M, Blaustein MP (1997) Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA 94:1800–1805. doi:10.1073/pnas.94.5.1800
Juhaszova M, Blaustein MP (1997) Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann NY Acad Sci 834:524–536. doi:10.1111/j.1749-6632.1997.tb52310.x
Daniel EE, El-Yazbi A, Cho WJ (2006) Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med 10:529–544. doi:10.1111/j.1582-4934.2006.tb00418.x
Shaul PW, Anderson RG (1998) Role of plasmalemmal caveolae in signal transduction. Am J Physiol 275:L843–L851
Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB (2005) The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288:H477–H485. doi:10.1152/ajpheart.00083.2004
James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB (1999) Identification of a specific role for the Na, K, -ATPase alpha-2 isoform as regulator of calcium in the heart. Mol Cell 3:555–563. doi:10.1016/S1097-2765(00)80349-4
Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB (2003) The alpha2 isoform of Na, K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278:53026–53034. doi:10.1074/jbc.M308547200
Adir Y, Welch LC, Dumasius V, Factor P, Sznajder JI, Ridge KM (2008) Overexpression of the Na-K-ATPase alpha2-subunit improves lung liquid clearance during ventilation-induced lung injury. Am J Physiol Lung Cell Mol Physiol 294:L1233–L1237. doi:10.1152/ajplung.00076.2007
Jorgensen PL (1974) Purification and characterization of (Na+ plus K+)-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta 356:36–52. doi:10.1016/0005-2736(74)90292-2
Skou JC, Esmann M (1979) Preparation of membrane-bound and of solubilized (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. The effect of preparative procedures on purity, specific and molar activity. Biochim Biophys Acta 567:436–444
Jorgensen PL, Skou JC (1971) Purification and characterization of (Na+ + K+)-ATPase. I. The influence of detergents on the activity of (Na+ + K+)-ATPase in preparations from the outer medulla of rabbit kidney. Biochim Biophys Acta 233:366–380. doi:10.1016/0005-2736(71)90334-8
Brotherus JR, Jost PC, Griffith OH, Hokin LE (1979) Detergent inactivation of sodium- and potassium-activated adenosinetriphosphatase of the electric eel. Biochemistry 18:5043–5050. doi:10.1021/bi00590a003
Santos HL, Lamas RP, Ciancaglini P (2002) Solubilization of Na, K-ATPase from rabbit kidney outer medulla using only C12E8. Braz J Med Biol Res 35:277–288
Jackson RL, Verkleij AJ, van Zoelen EJ, Lane LK, Schwartz A, van Deenen LL (1980) Asymmetric incorporation of Na+, K+-ATPase into phospholipid vesicles. Arch Biochem Biophys 200:269–278. doi:10.1016/0003-9861(80)90354-9
Morohashi M, Konishi K, Kawamura M (1988) Effects of Na+ and K+ on Artemia salina (Na, K)-ATPase solubilized with a zwitterionic detergent. J Biochem 103:1073–1077
Mandal A, Das S, Chakraborti T, Kar P, Ghosh B, Chakraboti S (2006) Solubilization, purification and reconstitution of Ca2+-ATPase from bovine pulmonary artery smooth muscle microsomes by different detergents: preservation of native structure and function of the enzyme by DHPC. Biochim Biophys Acta 1760:20–31
Kessi J, Poiree JC, Wehrli E, Bachofen R, Semenza G, Hauser H (1994) Short-chain phosphatidylcholines as superior detergents in solubilizing membrane proteins and preserving biological activity. Biochemistry 33:10825–10836. doi:10.1021/bi00201a033
Shivanna BD, Rowe ES (1997) Preservation of the native structure and function of Ca2+-ATPase from sarcoplasmic reticulum: solubilization and reconstitution by new short-chain phospholipid detergent 1, 2-diheptanoyl-sn- phosphatidylcholine. Biochem J 325:533–542
Chakraborti T, Ghosh SK, Michael JR, Chakraborti S (1996) Role of approtinin sensitive protease in the activation of Ca2+-ATPase by superoxide radical in microsomes of pulmonary vascular smooth muscle. Biochem J 317:885–890
Mandal M, Mandal A, Das S, Chakraborti S, Chakraborti T (2003) Identification, purification and partial characterization of tissue inhibitor of matrix metalloprotease-2 in bovine pulmonary artery smooth muscle. Mol Cell Biochem 254:275–287. doi:10.1023/A:1027389602772
Ikezu T, Trapp BD, Song KS, Schlega A, Lisanti MP, Okamoto T (1998) Caveolae, plasma membrane microdomains for α-secretase-mediated processing of the amyloid precussor protein. J Biol Chem 273:10485–10495. doi:10.1074/jbc.273.17.10485
Sargiacomo M, Sudol M, Tang ZL, Lisanti MP (1993) Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol 122:789–807. doi:10.1083/jcb.122.4.789
Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Vosatka AH, Tu YH, Cook RF, Sargiacomo M (1994) Characterization of caveolin reach membrane domains isolated from an endothelial-rich source: Implications for human disease. J Cell Biol 126:111–126. doi:10.1083/jcb.126.1.111
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354. doi:10.1073/pnas.76.9.4350
Chakraborti T, Das S, Chakraborti S (2005) Proteolytic activation of protein kinase C alpha by peroxynitrite in stimulating cytosolic phospholipase A2 in pulmonary endothelium: involvement of a pertussis toxin sensitive protein. Biochemistry 44:5246–5257. doi:10.1021/bi0477889
Lin H, Ozaki S, Fujishiro N, Takeda K, Imanaga I, Prestwich GD, Inoue M (2005) Subunit composition and role of Na+/K+-ATPases in adrenal chromaffin cells. J Physiol 564:161–172. doi:10.1113/jphysiol.2004.081455
Robinson JD (1967) Kinetic studies on a brain microsomal adenosine triphosphatase. Evidence suggesting conformational changes. Biochemistry 6:3250–3258. doi:10.1021/bi00862a034
Fiske CH, Subbaraw Y (1925) The colorimetric determination of Phosphorus. J Biol Chem 66:375–400
Hubert JJ, Schenk DB, Skelly H, Leffert HL (1986) Rat hepatic (Na+, K+)-ATPase: alpha-subunit isolation by immunoaffinity chromatography and structural analysis by peptide mapping. Biochemistry 25:4156–4163. doi:10.1021/bi00362a025
Deri Z, Adam-Vizi V (1993) Detection of intracellular free Na+ concentration of synaptosomes by a fluorescent indicator, Na+-binding benzofuran isophthalate: the effect of veratridine, ouabain, and α-latrotoxin. J Neurochem 61:818–825. doi:10.1111/j.1471-4159.1993.tb03592.x
Rigos CF, de Lima Santosh H, Thedei G Jr, Ward RJ (2003) Influence of enzyme conformational changes on catalytic activity investigated by circular dichroism spectroscopy. Biochem Mol Biol Educ 31:329–332. doi:10.1002/bmb.2003.494031050264
Karlish SJD (1980) Characterization of conformational changes in Na+/K+-ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr 12:111–136. doi:10.1007/BF00744678
Komatsu H, Guy PT, Rowe ES (1993) Effect of unilamellar vesicle size on ethanol-induced interdigitation in dipalmitoylphosphatidylcholine. Chem Phys Lipids 65:11–21. doi:10.1016/0009-3084(93)90077-G
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. doi:10.1016/0003-2697(85)90442-7
Daniel WW (1978) Biostatistics. In: Daniel WW (ed) A foundation for analysis in the health sciences (Estimation). Wiley, New York, pp 121–157
Juan G, Traganos F, Darzynkiewicz Z (1999) Histone H3 phosphorylation in human monocytes and during HL-60 cell differentiation. Exp Cell Res 246:212–220. doi:10.1006/excr.1998.4283
Garcia M, Bondada V, Geddes JW (2005) Mitochondrial localization of μ calpain. Biochem Biophys Res Commun 338:1241–1247. doi:10.1016/j.bbrc.2005.10.081
Eskelinen EL (2006) Role of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502. doi:10.1016/j.mam.2006.08.005
Hood JL, Brooks WH, Roszman TL (2004) Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and golgi apparatus. J Biol Chem 279:43126–43135. doi:10.1074/jbc.M408100200
Hood JL, Logan BB, Sinai AP, Brooks WH, Roszman TL (2003) Association of the calpain/calpastatin network with subcellular organelles. Biochem Biophys Res Commun 310:1200–1212. doi:10.1016/j.bbrc.2003.09.142
Baer HP, Vriend RA (1985) Cytosolic enzyme leakage from isolated smooth muscle preparations. Can J Physiol Pharmacol 63:164–165
Helenius A, McCaslin DR, Fries E, Tanford C (1979) Properties of detergents. Methods Enzymol 56:734–749. doi:10.1016/0076-6879(79)56066-2
Koepsell H (1986) Methodological aspects of purification and reconstitution of transport proteins from mammalian plasma membranes. Rev Physiol Biochem Pharmacol 104:65–137. doi:10.1007/BFb0031013
Silvius JR (1992) Solubilization and functional reconstitution of biomembrane components. Annu Rev Biophys Biomol Struct 21:323–348. doi:10.1146/annurev.bb.21.060192.001543
Santos HL, Ciancaglini P (2000) A practical approach to the choice of a suitable detergent and optimal conditions for solubilizing a membrane protein. Biochem Educ 28:178–182. doi:10.1016/S0307-4412(99)00107-7
le Maire M, Champeil P, Moller JV (2000) Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 1508:86–111. doi:10.1016/S0304-4157(00)00010-1
Tausk RJ, Karmiggelt J, Oudshoorn C, Overbeek JT (1974) Physical chemical studies of short-chain lecithin homologues. I. Influence of the chain length of the fatty acid ester and of electrolytes on the critical micelle concentration. Biophys Chem 1:175–183. doi:10.1016/0301-4622(74)80004-9
Tausk RJ, van Esch J, Karmiggelt J, Voordouw G, Overbeek JT (1974) Physical chemical studies of short-chain lecithin homologues. II. Micellar weights of dihexanoyl- and diheptanoyllecithin. Biophys Chem 1:184–203. doi:10.1016/0301-4622(74)80005-0
Hesketh TR, Smith GA, Houslay MD, McGill KA, Birdsall NJ, Metcalfe JC, Warren GB (1976) Annular lipids determine the ATPase activity of a calcium transport protein complexed with dipalmitoyllecithin. Biochemistry 15:4145–4151. doi:10.1021/bi00664a002
Anholt R, Lindstrom J, Montal M (1981) Stabilization of acetylcholine receptor channels by lipids in cholate solution and during reconstitution in vesicles. J Biol Chem 256:4377–4387
Fukuda K, Ikegami A, Nasuda-Kouyama A, Kouyama T (1990) Effect of partial delipidation of purple membrane on the photodynamics of bacteriorhodopsin. Biochemistry 29:1997–2002. doi:10.1021/bi00460a006
Esmann M (1988) Solubilization of Na+ , K+ -ATPase. Methods Enzymol 156:72–79. doi:10.1016/0076-6879(88)56010-X
Esmann M, Skou JC (1984) Kinetic properties of C12E8-solubilized (Na+-K+)-ATPase. Biochim Biophys Acta 787:71–80
de Foresta B, le Maire M, Orlowski S, Champeil P, Lund S, Moller JV, Michelangeli F, Lee AG (1989) Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in Ca2+-ATPase/lipid interaction. Biochemistry 28:2558–2567. doi:10.1021/bi00432a032
Ackers GK (1967) Molecular sieve studies of interacting protein systems.I. Equations for transport of associating systems. J Biol Chem 242:3026–3034
Brotherus JR, Jacobsen L, Jorgensen PL (1983) Soluble and enzymatically stable (Na+- K+)-ATPase from mammalian kidney consisting predominantly of protomer alpha beta-units Preparation, assay and reconstitution of active Na+, K+ transport. Biochim Biophys Acta 731:290–303. doi:10.1016/0005-2736(83)90021-4
de Lima Santos H, Lopes ML, Maggio B, Ciancaglini P (2005) Na, K-ATPase reconstituted in liposomes: effects of lipid composition on hydrolytic activity and enzyme orientation. Colloids Surf B Biointerfaces 41:239–248. doi:10.1016/j.colsurfb.2004.12.013
Cornelius F (1991) Functional reconstitution of the sodium pump. Kinetics of exchange reactions performed by reconstituted Na/K-ATPase. Biochim Biophys Acta 1071:19–66
Michelangeli F, Munkonge FM (1991) Methods of reconstitution of the purified sarcoplasmic reticulum (Ca2+–Mg2+)-ATPase using bile salt detergents to form membranes of defined lipid to protein ratios or sealed vesicles. Anal Biochem 194:231–236. doi:10.1016/0003-2697(91)90223-G
Acknowledgments
Financial assistance from the Department of Science & Technology (DST), Govt. of India and the Indian Council of Medical Research (ICMR), New Delhi are gratefully acknowledged. Thanks are due to Dr. N. Das and S. N. Dey (Indian Institute of Chemical Biology, Kolkata), Dr. A. N. Ghosh (National Institute of Cholera and Enteric Diseases, Kolkata), and Prof. Kasturi Datta (School of Environmental Sciences, Jawaharlal Nehru University, New Delhi) for their help in our research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, B., Chakraborti, T., Kar, P. et al. Solubilization, purification, and reconstitution of α2β1 isozyme of Na+/K+-ATPase from caveolae of pulmonary smooth muscle plasma membrane: comparative studies with DHPC, C12E8, and Triton X-100. Mol Cell Biochem 323, 169–184 (2009). https://doi.org/10.1007/s11010-008-9977-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9977-0