Abstract
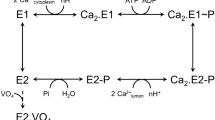
Ca2+ transport by sarcoplasmic reticulum Ca2+-ATPase involves ATP-dependent phosphorylation of a catalytic aspartic acid residue. The key process, luminal Ca2+ release occurs upon phosphoenzyme isomerization, abbreviated as E1PCa2 (reactive to ADP regenerating ATP and with two occluded Ca2+ at transport sites) → E2P (insensitive to ADP and after Ca2+ release). The isomerization involves gathering of cytoplasmic actuator and phosphorylation domains with second transmembrane helix (M2), and is epitomized by protection of a Leu119-proteinase K (prtK) cleavage site on M2. Ca2+ binding to the luminal transport sites of E2P, producing E2PCa2 before Ca2+-release exposes the prtK-site. Here we explore E2P structure to further elucidate luminal gating mechanism and effect of membrane perturbation. We find that ground state E2P becomes cleavable at Leu119 in a non-solubilizing concentration of detergent C12E8 at pH 7.4, indicating a shift towards a more E2PCa2-like state. Cleavage is accelerated by Mg2+ binding to luminal transport sites and blocked by their protonation at pH 6.0. Results indicate that possible disruption of phospholipid-protein interactions strongly favors an E2P species with looser head domain interactions at M2 and responsive to specific ligand binding at the transport sites, likely an early flexible intermediate in the development towards ground state E2P.
Similar content being viewed by others
Introduction
Sarco(endo)plasmic reticulum (SR) Ca2+-ATPase (expressed in adult fast-twitch skeletal muscle, SERCA1a), a representative member of P-type ion transporting ATPases, catalyzes Ca2+ transport coupled with ATP hydrolysis (Fig. 1) (for recent reviews, see Refs 1, 2, 3). The enzyme consists of three large cytoplasmic domains, Nucleotide binding (N), Phosphorylation (P), and Actuator (A), and ten transmembrane helices (M1~M10) (Figs 1 and 2). Ca2+ transport requires communication between the catalytic site on the cytoplasmic domains and the transport sites in the transmembrane helices via coupled structural changes, i.e. cytoplasmic domain motions and rearrangements of transmembrane helices. The enzyme is activated by the binding of two cytoplasmic Ca2+ ions at the high affinity transport sites composed of residues located on M4, M5, M6, and M8 (E2 to E1Ca2 in Fig. 1). Then it is auto-phosphorylated at the catalytic residue Asp351 with ATP to form an ADP-sensitive phosphoenzyme (E1P), which is capable of reacting with ADP to regenerate ATP in the reverse reaction. Upon E1P formation, the two bound Ca2+ are occluded in the transport sites (E1PCa2). The subsequent isomeric transition to the ADP-insensitive E2P form involves a large rotation of the A domain to associate with the P domain, thereby rearranging the Ca2+ binding sites to deocclude Ca2+, open the release path (luminal gate), and reduce the affinity, thus allowing Ca2+ release into the lumen. As a consequence, the catalytic site in E2P is prepared for subsequent aspartyl phosphate hydrolysis by tightening of associated A and P domains. In the first step towards hydrolysis, progressing from the ground state to the transition state, namely E2P + H2O → E2~P‡, the transport sites are protonated and the luminal gate closes tightly, preventing luminal Ca2+ access and driving the process forward4,5. The cytoplasmic part of the second transmembrane helix, M2, plays a crucial role in coupling A-domain motion and tilting of the P domain during the rearrangements of transport sites4,6,7,8,9.
The sequence is shown with intermediates and transition states (E1~PCa2ADP‡ and E2~P‡). Stable structural analog for each state developed with phosphate analogs BeF3−, AlF4− and MgF42− 4,6,17,19 is shown with gray-highlight. In the crystal structures E1Ca2·AlF4−·ADP and E2·BeF3− (PDB code: 2ZBD8 and 2ZBE8, respectively), the cytoplasmic domains A (yellow), P (cyan), and N (pink), M1~M10, occluded two Ca2+, and membrane position are indicated. Arrows on the domains in E1Ca2·AlF4−·ADP indicate their approximate motions to the E2·BeF3− structure to show changes in E1PCa2 → E2P+ 2Ca2+ as an available model.
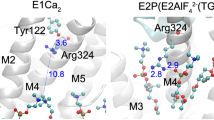
Structures E2·BeF3− with bound Mg2+ at the transport sites (formed at pH 7.0 and 50 mM Mg2+), E2·BeF3− with most probably protonated transport sites (formed at pH 5.7), and E2·BeF3−(TG) (PDB code: 3B9B11, 2ZBE8, 2ZBF8, respectively) are shown as a cartoon model. The cytoplasmic region indicated by the red broken line on the whole molecule of E2·BeF3− with bound Mg2+ is enlarged in the three top panels. In the three bottom panels, the view of transport sites from the luminal side as indicated by a large green arrow is shown. The A, P, and N domains and cytoplasmic part of M2 are yellow, cyan, pink, and purple, respectively. The Mg2+ and water molecules at the Ca2+ binding sites (transport sites) and Na+ bound at the K+ (Na+) site on the P domain are green, red, and blue spheres, respectively. The seven residues involved in the formation of Tyr122-hydrophobic cluster, Y122-HC (Leu119/Tyr122 on M2, Ile179/Leu180 on the A domain, Val705/Val726 on the P domain, and Ile232 on the A/M3-linker) are shown with van der Waals spheres, and colored green (Leu119/Tyr122), brown (Ile179/Leu180), and orange (Val705/Val726/Ile232). The BeF3− coordinated in the catalytic site behind the residues involved in Y122-HC is shown by a space-filling model (cyan for beryllium and purple for fluoride) and Asp351 (the auto-phosphorylation site) is shown in a ball-stick model in the panels (note that they are obscured by Y122-HC in E2·BeF3−(TG)). The Mg2+ bound to the catalytic site is not depicted as it is also hidden by Y122-HC. The TGES184 loop and Val200 loop (Lys189-Lys205) are colored by a red loop and a blue loop, respectively in all panels. The prtK-cleavage sites at Leu119 and Thr242 and the trypsin-cleavage sites at Arg198 and Arg505 are indicated (backbone carbon).
The E2P ground state, transition state (E2~P‡), and product complex (E2·Pi) in the E2P hydrolysis process are mimicked by the stable structural analogs E2·BeF3−, E2·AlF4−, and E2·MgF42−, respectively, as produced with the respective phosphate analogs for different configurational states4. Their crystal structures, without or with the potent inhibitor thapsigargin (TG), have been solved at atomic level7,8,10,11 following purification of the protein using a non-ionic detergent octaethylene glycol monododecyl ether (C12E8). Commensurate with the structural changes mentioned, the crystal structures are subtly different although the overall molecular structure of the compactly organized cytoplasmic A, P, and N domains with tightly bound BeF3− and occluded Mg2+ at the catalytic site and the arrangement of transmembrane helices are similar. Namely, the E2·BeF3− crystal produced at pH 7.0 in 50 mM Mg2+ has wide open transport sites (luminal gate open) with one bound Mg2+11 and that at pH 5.7, where the transport sites are protonated and Mg2+ is absent, the luminal access pathway is less open8 (Fig. 2). The structures with bound TG at a cavity surrounded by M3, M5, and M7, namely E2·BeF3−(TG), and those of E2·AlF4−(TG) and E2·MgF42−(TG), are different again, and the luminal gate is tightly closed. The closure is associated with formation of hydrophobic interaction network, the Tyr122-hydrophobic cluster (Y122-HC) by Leu119/Tyr122 on the cytoplasmic part of M2 and five residues of the gathered A and P domains (Ile179/Leu180 (A), Val705/Val726 (P)) and A/M3-linker (Ile232 on the loop connecting the A domain with M3). Significantly, in the E2·BeF3− crystals without TG, where the gate is open, the side chains of Leu119/Tyr122 are close but pointing away from the other gathered five residues, indicative of weaker domain interactions here (Fig. 2).
Extensive mutation and kinetic studies have demonstrated12,13,14,15 that all seven residues involved in Y122-HC including Leu119/Tyr122 are crucial for opening the gate, reducing Ca2+ affinity, and allowing rapid Ca2+-release (E2PCa2 → E2P + 2Ca2+), and for subsequent gate-closure and the formation of a catalytic site with hydrolytic ability. Investigation of the structural changes during these events has been aided by proteolytic digestion patterns, including a prtK site at Leu119 4,16,17. The site is exposed in the unphosphorylated E2 form but protected in E2·BeF3−, E2·AlF4−, and E2·MgF42− as well as in the TG-bound forms of these analogs. Thus susceptibility to prtK attack or otherwise seems a good indicator of the state of the gathering of the head domains on M2. Significantly, E2PCa2 an early E2P species, is uniquely susceptible to attack, an indication of a loose arrangement of head domains on M2 prior to progression to ground state E2P4,18,19.
Unexpectedly, we now find that low, non-solubilizing concentrations of C12E8 render the prtK site at Leu119 in E2P (E2·BeF3−) susceptible to attack. It is as though the detergent has released constraints at the transmembrane helices to favor a state closer to that on Ca2+ binding to the luminal sites, namely E2PCa2. The phenomenon uncovers a hitherto undescribed intermediate just prior to ground state E2P, stabilized by detergent that is uniquely susceptible to diverse ligand binding and cross-protein conformational changes. It shows that phospholipid-protein interactions directly participate the conformational changes associated with luminal gating events and expedite Ca2+ release.
Results
PrtK-cleavage of Leu119-site in E2·BeF3− with C12E8 at pH 7.4
In Fig. 3a, prtK-proteolysis of E2·BeF3− is performed at pH 7.4 in 0.1 M K+ without and with a non-solubilizing low concentration of C12E8. In the absence of C12E8, E2·BeF3− is completely resistant to prtK both without and with A23187 as found previously4. In the presence of C12E8, a 95-kDa fragment (p95) is produced by specific prtK-cleavage at the Leu119-site without any other cleavages. Cleavage is accelerated by 30 mM Mg2+, but no cleavage occurs in the absence of C12E8 even at 30 mM Mg2+. In Fig. 3d, the Mg2+ concentration dependence of the specific prtK-cleavage rate at the Leu119-site is determined in C12E8 and different monovalent cations (K+, Na+, and Li+) at 0.1 M. The rate increases with increasing Mg2+ concentration – binding to a low affinity site favors exposure. The cleavage is faster in Na+ and K+ as compared with that in Li+ or in the absence of monovalent cation, thus K+ or Na+ binding at the K+ site on the P domain20,21 increases prtK attack at Leu119.
The proteolysis was performed for various times with prtK and trypsin as indicated with E2·BeF3− (a,e), E2·AlF4− (b,f), and E2·MgF42− (c,f) of SR vesicles in the presence or absence of 0.15 mg/ml C12E8 or 15 μM A23187 in 50 mM MOPS/Tris pH 7.4 (a–c) or MES/Tris pH 6.0 (e,f), 0.1 M KCl, 1 mM EGTA, and 0 or 30 mM MgCl2 without or with 4 μM TG (“TG”), as indicated. The “E2·TG” state of SR vesicles un-treated with the metal fluoride was subjected to the proteolysis as a control. In (d), the rate of prtK digestion of 110 kDa-ATPase chain in C12E8 at pH 7.4 was determined at various concentrations of MgCl2 in 0.1 M KCl, NaCl, or LiCl or in the absence of these salts, otherwise as in (a) and as described under “METHODS”. The fragments indicated on the right of a panel are p95 produced by the prtK-cleavage at the Leu119-site on M2, p81/p83 produced by the prtK-cleavage at the Thr242-site on A/M3-linker (p83) and Ala746 on M5 (p81)16,39, and the tryptic A1 fragment produced by cleavage at the Arg198-site on the A fragment (N-terminal half), which is formed very rapidly together with the B fragment (C-terminal half) by cleavage at Arg505-site40.
E2·BeF3− cleavage in C12E8 is inhibited by thapsigargin (TG), which binds tightly to a cavity surrounded by M3, M5, and M7, fixing the arrangement of transmembrane helices with a tightly closed luminal gate22,23 (Fig. 3a “C12E8+ TG” for E2·BeF3−). On the other hand, in the BeF3−-free state with bound TG (“E2·TG”) with Mg2+ as well as without Mg2+, the 110-kDa ATPase chain is very rapidly cleaved producing p95 and p81/p83 fragments by cleavages at Leu119 and at Thr242 (p83) and Ala746 (p81), respectively, in agreement with previous findings16.
Tryptic T2 (Arg198)-site in E2·BeF3− is completely resistant in C12E8
The association of the Val200 loop (Lys189-Lys205) on the A domain with the P domain by ionic interactions is crucial for E2P structure formation and occurs as a consequence of the A domain’s large rotation during the E1PCa2 → E2P isomeric transition6,7,24. With the changes, the Arg198-tryptic T2 site in this loop becomes completely resistant to tryptic attack4,6. In Fig. 3a, the trypsin proteolysis was performed as described above with prtK. In the BeF3−-free state with bound TG as a control (“E2·TG”) in which the A and P domains are not fixed, the Arg198-site is cleaved producing the A1 and A2 fragments (the A2 fragment is not seen because it is at the gel front) as found previously6. In E2·BeF3−, the A1 and A2 fragments are not produced regardless of the presence of C12E8 and 30 mM Mg2+, thus the Arg198-site is completely resistant, consistent with association of the A and P domains by an ionic network as seen in the E2·BeF3− crystal structures8,11.
E2·BeF3− in C12E8 is completely resistant to prtK at pH 6.0
In Fig. 3e, prtK-proteolysis was performed at pH 6.0 otherwise as in Fig. 3a. At this pH the luminal transport sites are expected to be protonated. No cleavage of the 110 kDa-ATPase chain occurred even in C12E8 and 30 mM Mg2+. The tryptic Arg198-site was also completely resistant at pH 6.0 as at pH 7.4 without and with C12E8 and 30 mM Mg2+.
E2·AlF4− and E2·MgF42− are completely resistant to prtK even in C12E8 at pH 7.4 and 6.0
E2·AlF4−, the analog for the transition state E2~P‡ is completely resistant to prtK at pH 7.4 and 6.0 even in the presence of C12E8 both without and with 30 mM Mg2+ (Fig. 3b,f). The Arg198-site is also protected from trypsin in all these conditions. E2·MgF42−, the analog for the product complex (E2·Pi) is completely resistant to prtK and to trypsin in all these conditions as E2·AlF4− (Fig. 3c,f).
Hydrophobic nature of the nucleotide/catalytic site revealed by TNP-AMP superfluorescence
TNP-AMP binds to the ATP binding site with a very high affinity and develops an extremely high “superfluorescence” in the E2P ground state and its analog E2·BeF3−4,25. The TNP moiety binds at the adenine position in the N domain and the superfluorescence can be ascribed to a favorable TNP moiety Phe487 interaction and site-occlusion that excludes non-specific water and increases hydrophobicity by the contribution of Arg174 on the A domain at the A-N interface on the TNP binding pocket26. The superfluorescence is completely lost during E2P + H2O → E2~P‡, as demonstrated with the change E2·BeF3− → E2·AlF4− 4, probably through TNP-Phe487 mal-alignment and water influx here. In Fig. 4, the superfluorescence development in E2·BeF3− upon the TNP-AMP binding at saturating 4 μM was examined without and with C12E8 at pH 7.4 and 6.0 and various concentrations of Mg2+ in 0.1 M K+ or Li+. There was almost no effect of C12E8 on superfluorescence development. Specific K+ binding on the P domain20,21 also had virtually no effect (compare the data in K+ with those in Li+). Increasing Mg2+ concentration up to 60 mM caused only slight decrease. The results show that the catalytic/nucleotide site, starting from the E2P ground state, is not affected by C12E8, Mg2+, K+, and protonation of transport sites.
E2·BeF3− or the BeF3−-free Ca2+-ATPase (E2) in SR vesicles (0.06 mg protein/ml) were incubated at 25 °C for 3 min in 0.5 mM EGTA, 30 mM MES/Tris (pH 6.0) or MOPS/Tris (pH 7.4), 0.1 M KCl or LiCl, and 0–60 mM MgCl2 with or without 0.02 mg/ml C12E8 and/or 2.5 μM A23187, as indicated in the figure. Subsequently, TNP-AMP at saturating 4 μM was added. The fluorescence intensity was obtained by subtracting the protein background level without TNP-AMP and the level of 4 μM TNP-AMP without SR vesicles, and plotted versus Mg2+ concentration.
E2·BeF3− in C12E8 and Mg2 +is resistant to luminal Ca2+-induced reverse conversion to E1Ca2·BeF3−
The E2P ground state possesses luminally partially open low affinity transport sites and luminal Ca2+ at sub-mM to ~mM concentration is able to bind and cause reverse isomerization E2P + 2Ca2+ → E2PCa2 → E1PCa2, which contributes to the proper setting of luminal Ca2+ concentration through “back-door inhibition”. This reverse process as well as the forward EP isomerization is mimicked and characterized with the structural analogs E2·BeF3− (E2P), E2·BeF3−·Ca2 (E2PCa2, the transient intermediate state before the Ca2+-release), and E1Ca2·BeF3− (E1PCa2)4,17,18,19. In Figs 5 and 6, the effect of luminal Ca2+ on E2·BeF3− was examined at pH 7.4 in C12E8 or A23187, various concentrations of Mg2+, and 0.1 M K+ or Li+. Here it should be noted that the E1Ca2·BeF3− complex is not stable and rapidly decomposes to E1Ca2 in the presence of a high concentration of Ca2+ (due to Ca2+-substitution at the unoccluded catalytic Mg2+ site in E1Ca2·BeF3−17), on the other hand, it is very rapidly isomerized to E2·BeF3− releasing Ca2+ upon the removal or reduction of luminal free Ca2+ concentration (to below ~100 μM) as the process mimics the isomeric transition E1PCa2 → E2P + 2Ca2+17. Also, the E1Ca2·BeF3− complex decomposes to E1Ca2 upon ADP binding, mimicking the ADP-induced reverse dephosphorylation of E1PCa2, and upon TNP-AMP binding probably analogous to the ADP-induced process, in contrast to a stable E2·BeF3− with bound ADP or TNP-AMP17.
E2·BeF3− in SR vesicles was incubated at 25 °C for 3 min in 30 mM MOPS/Tris (pH 7.4), 0.1 M KCl or LiCl, 0.5 mM EGTA, 0–60 mM MgCl2 with or without 0.15 mg/ml C12E8 or 15 μM A23187, as indicated. Subsequently Ca2+ was added to give 0.5 mM free concentration and incubated for various times, then diluted 10-fold with the above solution containing 5 mM EGTA without Ca2+. At 30 s after dilution, 4 μM TNP-AMP was added to determine the superfluorescence intensity. The representative time courses of loss of superfluorescence in C12E8 in 0.1 M K+ are shown in inset. The rate of Ca2+-induced E2·BeF3− decomposition was determined by least-squares fit of a single exponential to the time course and plotted versus the Mg2+ concentration.
(a) E2·BeF3− in SR vesicles was incubated without or with 0.15 mg/ml C12E8 or with 15 μM A23187 at 25 °C for 3 min in 30 mM MOPS/Tris (pH 7.4), 0.1 M LiCl, 0.5 mM EGTA, and 0 (upper panel) or 30 mM MgCl2 (lower panel), then Ca2+ was added to give 0.5 mM free concentration. After 10 s, prtK was added at 0.5 mg/ml and incubated for indicated times. As a control, the BeF3−-free Ca2+-ATPase in SR vesicles (“E1Ca2”) was subjected to the proteolysis in 0.5 mM free Ca2+. (b) The prtK proteolysis was performed under the conditions that produce and perfectly stabilize E1Ca2·BeF3− 17, i.e. 30 mM MOPS/Tris (pH 7.0), 0.1 M KCl, 15 mM MgCl2, and 0.7 mM CaCl2 in the presence of 100 μM BeCl2 and 2 mM KF without and with 15 μM A23187, and the effect of C12E8 was examined by including C12E8 without A23187, otherwise as in (a). The BeF3−-free Ca2+-ATPase (“E1Ca2”) in A23187 and in C12E8 was subjected to proteolysis otherwise as above. Note that the slow decomposition of E2·BeF3− in Ca2+ in the absence of A23187 and C12E8 (a) is probably due to slow Ca2+ permeation into the SR vesicles lumen17. (c) E1Ca2·BeF3− was produced by incubating SR vesicles for 30 min with 100 μM BeCl2 and 2 mM KF in the absence of A23187 and C12E8 otherwise as in (b), then C12E8 or A23187 was added to give 0.02 mg/ml and 2.5 μM, respectively. At 10 s after this addition, TNP-AMP was added to give a saturating 4 μM, and the fluorescence monitored; trace b, without C12E8 and A23187; traces c and d, in A23178 and in C12E8, respectively. Trace e, the fluorescence monitored with E2·BeF3− in the presence of 2 mM EGTA without adding Ca2+. Trace a, the non-superfluorescent E1Ca2 level (BeF3−-free Ca2+-ATPase) in 4 μM TNP-AMP.
In Fig. 5, taking these known properties into account, we first determined the overall time course of the Ca2+-induced E2·BeF3− reverse conversion and decomposition to E1Ca2 (E2·BeF3− + 2Ca2+ → E2·BeF3−·Ca2 → E1Ca2·BeF3− → E1Ca2) by adding an excess EGTA after various times of incubation with 0.5 mM Ca2+ thereby converting the remaining E1Ca2·BeF3− to the stable E2·BeF3− species, and in addition adding TNP-AMP to determine superfluorescence development to estimate the total amount of E2·BeF3− and E1Ca2·BeF3− species remaining at the time of EGTA addition. In Fig. 6, prtK proteolysis was performed for a short period during the 0.5 mM Ca2+ incubation and without the EGTA addition to identify the structural states of EP species under representative conditions in Fig. 5 (although the Ca2+-induced process proceeds).
First in Fig. 5 where TNP-AMP superfluorescence is examined, we found both with K+ and without K+ (with LiCl) that the Ca2+-induced reverse conversion/decomposition of E2·BeF3− is considerably slower in C12E8 than in A23187, and increasing Mg2+ to ~20 mM in C12E8 causes a marked retardation or almost complete inhibition. The retardation by Mg2+ in C12E8 is much stronger and occurs at much lower Mg2+ concentration than in A23187. In the absence of both C12E8 and A23187, i.e. with an impermeable SR membrane, no conversion nor decomposition of E2·BeF3− occurs with Ca2+, therefore the Ca2+-induced decomposition is due to the Ca2+ access from the luminal side as found previously4,17. Regarding the K+ effect, the luminal Ca2+-induced conversion/decomposition of E2·BeF3− is considerably faster in K+ than in its absence, therefore specific K+ binding20,21 accelerates the process.
Then in Fig. 6a, prtK-proteolysis was performed to identify the structural state stabilized in C12E8 with, most typically, 30 mM Mg2+ in the absence of K+ during luminal Ca2+-induced E2·BeF3− reverse conversion and decomposition. Here, the sample was incubated first with 0.5 mM Ca2+ for 10 s, and then with a high concentration of prtK for various times without removal of Ca2+. The proteolytic pattern was compared with those of BeF3−-free E1Ca2 and of E1Ca2·BeF3− that is formed and stabilized perfectly under the previously identified most appropriate conditions, i.e. at pH 7.0 with 0.7 mM Ca2+ and 15 mM Mg2+ in 0.1 M K+ in the absence or presence of A23187 17; in these states, p81/p83 fragments are produced due to cleavage at Thr242 (p83) and Ala746 (p81) without production of the p95-fragment (Fig. 6b). In C12E8 and Ca2+ (Fig. 6a), E2·BeF3− both without and with 30 mM Mg2+ is degraded slowly as compared with E1Ca2, producing the stable p95 fragment as seen with E2·BeF3− in C12E8 without Ca2+ (cf. Fig. 3) and a small amount of p81/p83 fragments, which degrade rapidly as the BeF3−-free E1Ca2 state. Note also that the 110-kDa ATPase chain degradation is much slower and formation of the rapidly degrading p81/p83 fragments is much less in 30 mM Mg2+ than without Mg2+. The results show that E2·BeF3− in C12E8 and Ca2+ is resistant to the luminal Ca2+-induced reverse conversion to E1Ca2·BeF3−, which can be interpreted as very slow Ca2+ binding to luminal transport sites and what slow conversion occurs is markedly retarded by 30 mM Mg2+. These results accord with those using superfluorescence as the indicator in Fig. 5.
In the presence of A23187, as seen in Fig. 6a, formation of the p81/p83 fragments from E2·BeF3− in Ca2+ occurs without any p95 fragment, as with E1Ca2 and E1Ca2·BeF3− in A23187 (cf. Fig. 6b) indicating a fast conversion of E2·BeF3− to E1Ca2·BeF3− without the detergent and with the ionophore. These results together with the retardation by Mg2+ of loss of TNP-AMP superfluorescence (Fig. 5) indicate that E1Ca2·BeF3− is formed from E2·BeF3− without detergent on luminal Ca2+ binding and further decomposed to E1Ca2, and that Mg2+ at a high concentration retards the decomposition of E1Ca2·BeF3− to E1Ca2 probably by inhibiting the Ca2+-replacement of Mg2+ at the unoccluded catalytic subsite17.
Forward conversion of E1Ca2·BeF3− to E2·BeF3− is favored in C12E8
Also in Fig. 6b, it can be seen that under conditions where E1Ca2·BeF3− is perfectly stable in A2318717, the addition of C12E8 in place of A23187 produces the same proteolytic pattern as developed with E2·BeF3− in C12E8 and Ca2+. The results reveal that the E2·BeF3− state is produced and stabilized in C12E8 even under conditions that perfectly stabilize E1Ca2·BeF3− in the absence of C12E8. This was further verified by superfluorescence development and loss upon TNP-AMP addition in Fig. 6c, which was performed on the basis of previous findings17 that E1Ca2·BeF3− rapidly decomposes to the non-fluorescent E1Ca2 state upon TNP-AMP binding whereas E2·BeF3− with bound TNP-AMP is stable, and also that the superfluorescence intensity is greater in E2·BeF3− than in E1Ca2·BeF3− (by approximately 25%). In Fig. 6c, E1Ca2·BeF3− was first formed under the conditions in Fig. 6b without A23187 and C12E8, and then A23187 or C12E8 added. After 10 s, superfluorescence upon TNP-AMP addition was recorded. In A23187 or in its absence, superfluorescence development is followed by its rapid loss, which is due to E1Ca2·BeF3− decomposition to E1Ca2 on TNP-AMP binding17. In C12E8, greater superfluorescence develops and its loss is considerably slower than in A23187. The results show again that in C12E8, E2·BeF3− is formed even under conditions that perfectly stabilize E1Ca2·BeF3− (although E2·BeF3− is slowly decomposed to the non-fluorescent E1Ca2 state via E1Ca2·BeF3− in high Ca2+ and decomposition by TNP-AMP).
E2P hydrolysis
In Fig. 7, the effects of C12E8, K+, and Mg2+ on the forward E2P hydrolysis rate were examined at pH 7.4 and 6.0. Here E2P was first formed in the reverse reaction of hydrolysis from the Ca2+-deprived E2 state and 32Pi in 7 mM Mg2+ without or with C12E8 (or with A23187) in 20% (v/v) Me2SO, conditions that favor E2P formation. Then hydrolysis was initiated by a 20-fold dilution in non-radioactive Pi, various concentrations of Mg2+, and 0.1 M K+ (Fig. 7a) or Li+ (Fig. 7b) at the desired pH. In K+ at pH 7.4, C12E8 markedly retards hydrolysis as found previously at pH 7.527, and increasing Mg2+ concentration in C12E8 hardly affects the rate (perhaps a slight increase), but the cation decreases the rate in the absence of C12E8. Because this decrease is observed both without and with A23187 (an ionophore for Ca2+ and Mg2+) and because Me2SO (used for the Pi-induced E2P formation) does not permeabilize the SR membrane, the hydrolysis reaction rate itself is likely affected by Mg2+ at the cytoplasmic side. At pH 6.0 in K+, hydrolysis is much slower than at pH 7.4, as is well known28, and C12E8 and Mg2+ have almost no effect on the slowed rate.
SR vesicles were phosphorylated with 0.1 mM 32Pi at 25 °C for 10 min in 5 μl of a mixture containing 0.3 mg protein/ml with or without 3 μM A23187 as indicated, 1 mM EGTA, 7 mM MgCl2, 30 mM MOPS/Tris (pH 7.4) or MES/Tris (pH 6.0), and 20% (v/v) Me2SO. The mixture was then cooled, and a small volume of C12E8 was added to give 0.1 mg/ml (1/3 (w/w) of the protein) to the indicated samples. Subsequently, the samples were diluted at 0 °C by the addition of 95 μl of a mixture containing 0.1 mM non-radioactive Pi, 105 mM KCl (a) or LiCl (b), 1 mM EGTA, 1–30 mM MgCl2, and 50 mM MOPS/Tris (pH 7.4) or MES/Tris (pH 6.0), as indicated with different symbols. The E2P hydrolysis rate was determined as described under “METHODS” and plotted versus Mg2+concentration. Note the difference in the scale of the ordinate in (a) and (b).
In the absence of K+ (Fig. 7b), E2P hydrolysis at both pH 7.4 and 6.0 is much slower than in 0.1 M K+ (by ~10-fold at the respective pH), in agreement with the well-known acceleration of hydrolysis by specific K+ binding on the P domain20,21. In the absence of K+, hydrolysis in C12E8 is only slightly slower than that without C12E8. Mg2+ at ~10 mM somewhat increases the rate although the rate is still much slower than that in the presence of K+. In summary, induction of the detergent-stabilized state strongly inhibits hydrolysis at pH 7.4, but not following protonation of the transport sites at pH 6.0, and only in the presence of K+.
Discussion
Ca2+ transport by Ca2+-ATPase includes phosphorylated intermediates where Ca2+ is occluded at the transport sites and then released to the lumen, i.e. E1P[Ca2] → E2P + Ca2+. During this process the A domain swings around and engages with the P domain and neck region of the protein at the cytoplasmic part of M2 (Fig. 1). The A-domain rotation inclines the P-domain by pulling an A/M1′-link, pushing M4 down towards the lumen to release the Ca2+ 8,18,19. There is evidence that the gathering and interaction of A and P domains at the cytoplasmic part of M2 occurs progressively. Namely, changes, which are linked to deocclusion and opening of the luminal access channel with an affinity reduction, are followed by constrictions to limit access, protonation, and finally closure, and all these changes are synchronized with catalytic site preparations for hydrolysis4,13,15,17,18,19. Part of the development is seen with the Leu119 prtK cleavage site, being exposed in E2PCa2, hidden in E2P, E2~P‡ and E2·Pi, and exposed again in E24,18,19. We found here that non-solubilizing concentrations of C12E8 uncovers the Leu119 prtK site of E2P, as depicted in its analog E2·BeF3−. This indicates that membrane perturbation drives the intermediate towards one more like that with bound Ca2+, and points to an earlier catalytic intermediate with a looser arrangement in the head region, as expected for early engagement of the rotated A domain. The responsiveness of E2P to membrane perturbation and the detergent-induced state to ligand binding (Ca2+, Mg2+, K+, H+, and TG) through changes in exposure of the Leu119 prtK site at the cytoplasmic part of M2 points to flexible and rather unstable forms. These properties are due most probably not only to its unoccupied transport sites and associated circle of negative charges, but also to a loose meeting of domains and neck region with largely unsecured interactions at the cytoplasmic part of M2. The downward thrust of M4 (by a full turn of an α-helix8), together with M3, is probably partly stabilized by surrounding phospholipids and insertion of non-ionic detergent between them could be disruptive. In the head region the interactions at Leu119 involve the formation of Y122-HC, a hydrophobic interaction network of Tyr122/Leu119 with the A and P domains and A/M3-linker involving seven residues (Fig. 2). As mentioned above, the interactions are likely progressive, loose at first as the A domain engages followed by incremental tightening in E2P to the fully stabilized state in E2~P‡ and E2·Pi. Indeed, in the E2·BeF3− crystal structures (formed in the presence of C12E8) with the bound Mg2+ or with protonation without the Mg2+, Leu119/Tyr122 on M2 are close but not yet associated with the five other gathered residues involved in Y122-HC formation. The knitting of Leu119 and Tyr122 with the other residues is seen in the crystal structures of analogs of the next intermediates, E2~P‡ and E2·Pi. Accumulating interactions fit perfectly with the staggered changes at the luminal transport sites, from closed to open to closed again.
Stabilization of the early detergent-induced state is seen in the forward direction of catalysis coming from E1PCa2 (E1Ca2·BeF3−) and in the backward direction with Ca2+ binding to the luminal sites of E2P (E2·BeF3−), using both TNP-AMP superfluorescence and the prtK sites as probes. Our results suggest that the E2·BeF3− structural state favored in C12E8 and stabilized by Mg2+ represents one between E1PCa2 and Ca2+-released E2P, i.e. the transient E2P state immediately following Ca2+ release denoted as E2P∗ with luminally open and vacant low affinity transport sites (E2P∗Ca2 → E2P∗ in Fig. 8). C12E8 stabilizes the E2P∗ state and thereby retards both the luminal Ca2+-induced reverse conversion and the forward hydrolysis of E2P at pH 7.4. Mg2+ binding probably prevents luminal Ca2+-access and consequent reverse conversion (Figs 3,5 and 6). This Mg2+ is likely at or near the luminally open Ca2+ transport sites (in addition to Mg2+ occluded at the catalytic subsite in E2·BeF3− and E2P) as actually seen in the E2·BeF3− crystal produced in a high concentration of Mg2+11. The Mg2+ probably manifests itself in the competitive inhibition by Mg2+ of luminal Ca2+-induced reverse isomerization E2P+ 2Ca2+ → E1PCa229. Notably also, the dephosphorylated E1 state is able to accommodate one Mg2+ at the transport sites and forms E1·Mg, which favors high affinity Ca2+-binding resulting in a rapid E2 → E1·Mg → E1Ca2 transition30,31 (Fig. 1). Thus it seems that Mg2+ binds at the empty transport sites both in the unphosphorylated and phosphorylated states and modifies transport function.
The effects of C12E8, luminal Ca2+, and Mg2+ found in this study are summarized. E2P[Ca2] (E2P with occluded Ca2+) and ∗E2PCa2 (E2P with luminally open gate and with bound Ca2+ yet at a high affinity) were previously identified by the elongation of the A/M1′-linker18,19 and by substitutional mutation of Leu119 and Tyr12215, but they are transient intermediates and have never been trapped or identified in wild type15,18,19; therefore they are shown in brackets. The E2P structural states found in this study at pH 7.4, the Leu119-cleavable state and the prtK-resistant state are denoted as E2P∗ and E2P, respectively. The prtK-resistant state found in C12E8 at pH 6.0 (i.e. with protonation of the transport sites) is denoted as E2P(∗). Note that Y122-HC formation on gathering of Leu119/Tyr122 on M2 with engaged A and P domains occurs progressively during E2P processing and couples with luminal gating (see more in “Discussion”).
The E2·BeF3− structures revealed in C12E8 and in A23187 at pH 7.4 reflect E2P∗ and E2P respectively in Fig. 8 on the basis of prtK-resistance. Analysis of the Mg2+ inhibition of luminal Ca2+-induced reverse conversion of E2·BeF3− in Figs 5 and 6 indicates that Mg2+ accesses E2P∗ with a much higher affinity than E2P. Thus the transport sites appear more open and accessible to Mg2+ on the luminal side in the Leu119-site cleavable E2P∗ state than in the prtK-resistant E2P ground state. In fact, in the E2·BeF3− crystal with bound Mg2+ at the transport sites, the sites are actually more open to the lumen than in the structure without Mg2+ (Fig. 2). Note also that in E2·AlF4− and E2·MgF42− (E2~P‡ and E2·Pi) and in E2·BeF3− with bound TG, Ca2+ cannot bind as the luminal gate is tightly closed4,7,8, and the Leu119-site is completely resistant to prtK regardless of the presence of C12E8 (Fig. 3). These findings suggest that the structural change reflected by prtK resistance at Leu119 is associated with luminal gating, supporting the above conclusion that substantial luminal gate closure occurs in E2P∗ → E2P, which probably involves gathering of Leu119/Tyr122 with the engaged A and P domains to accomplish the Y122-HC network. Then the passage is completely sealed in E2~P‡ and E2·Pi (E2·AlF4− and E2·MgF42−)4.
Previous kinetic analysis of the luminal Ca2+-induced reverse isomerization E2P + 2Ca2+ → E1PCa2 indicated14 that the luminal Ca2+ access to the transport sites in E2P is rate-limiting. This is described in Fig. 8 with the equilibrium E2P∗ ↔ E2P, where the former state is more open and the latter relatively closed. This view agrees with our finding on the Ca2+ release kinetics E1PCa2 → E2PCa2 → E2P+ 2Ca2+ 15 that the E2P structure proceeds from a luminally open state for Ca2+ release (corresponding to E2P∗ in Fig. 8) to a closed state (E2P) with the structural contribution of Leu119/Tyr122. The observation that Mg2+ hardly alters the forward E2P hydrolysis rate in C12E8 (Fig. 7a) can be accounted for by a rapid Mg2+ binding/release relative to the hydrolysis reaction process, and implies that Mg2+ binding favors the forward reaction.
At pH 6.0 in which the transport sites are protonated, the Leu119-site is completely resistant to prtK regardless of the presence of C12E8, and the E2P hydrolysis rate is not affected by C12E8. In Fig. 8, the protonated structural state with the prtK-resistance revealed in C12E8 is denoted as E2P(∗) to be discriminated from the prtK-cleavable E2P∗ state without protonation. Protonation neutralizes charges at the Ca2+-binding sites and stabilizes the arrangement of transmembrane helices via a hydrogen bonding network8, which lowers Ca2+-accessibility (without completely closing the gate as seen in the E2·BeF3− crystal formed at pH 5.78). The protonated state proceeds promptly to subsequent hydrolysis with tight gate closure E2P+ H2O → E2~P‡ (E2·BeF3− → E2·AlF4−), as indicated previously by kinetic analysis of E2P hydrolysis5.
K+ in the presence of C12E8 accelerates both forward E2P hydrolysis and luminal Ca2+-induced reverse conversion of E2·BeF3− (Figs 5 and 7). These findings are in complete agreement with the known role of specific K+ binding on the P domain in accelerating both forward hydrolysis20,21 and luminal Ca2+-induced reverse conversion of E2P14. K+ binding likely destabilizes both E2P and E2P∗ in Fig. 8, thus promoting rapid transport.
Finally, induction of the detergent-stabilized state, an early intermediate to ground state E2P, shows how phospholipids are intimately involved in the latter’s stabilization. Membrane perturbation effects during the transport cycle may be under-appreciated as fundamental to the mechanism.
Methods
Preparation of SR vesicles and treatment with BeFx, AlFx, and MgFx
SR vesicles were prepared from rabbit skeletal muscle as described32,33, in which all the methods were carried out in accordance with institutional laws and regulations of the Asahikawa Medical University and the experimental protocols were approved by the Animal Experimentation Ethics Committee of the Asahikawa Medical University (license number 16006). The content of the phosphorylation site in the vesicles and the Ca2+-dependent ATPase activity were determined as described32,33. E2·BeF3−, E2·AlF4−, and E2·MgF42− were produced by incubating the SR vesicles with the respective metal fluoride and by washing the unbound ligands as described previously4.
Formation and hydrolysis of E2P
The SR vesicles were phosphorylated with 0.1 mM 32Pi at 25 °C for 10 min in 20% (v/v) Me2SO in the absence of Ca2+, after which the samples were cooled and diluted 20-fold by a solution containing 2.1 mM non-radioactive Pi to initiate the hydrolysis of 32Pi-labeled E2P, otherwise as described in detail in the legend to Fig. 7. The reaction was quenched with ice-cold trichloroacetic acid containing Pi. The precipitated proteins were separated by 5% SDS-PAGE at pH 6.0 according to Weber and Osborn34. The radioactivity associated with the separated Ca2+-ATPase was quantified by digital autoradiography as described35. Rapid kinetics measurement of hydrolysis was performed with a handmade rapid mixing apparatus and the rate of hydrolysis was determined with the least-squares fit to a single exponential, as described35.
Proteolytic analysis
SR vesicles (0.45 mg/ml protein) were subjected to proteolysis at 25 °C by addition of trypsin (at 0.3 mg/ml, L-1-tosylamido-2-phenylethyl chloromethyl ketone-treated) or proteinase K (prtK, at 0.1 mg/ml, Sigma) as described previously6,16, otherwise as indicated in the figure legends. The proteolysis was terminated by trichloroacetic acid, and the samples were subjected to Laemmli SDS-polyacrylamide gel electrophoresis36, and densitometric analyses of the gels stained with Coomassie Brilliant Blue R-250, as described6,16. The degradation rate of 110-kDa ATPase chain with prtK was determined by least-squares fit of a single exponential to the time course (0–150 min) as described previously16.
Fluorescence measurements
The TNP-AMP fluorescence of the Ca2+-ATPase (0.06 mg/ml protein, TNP-AMP from Molecular Probes® Life Technologies) was measured on a RF-5300PC spectrofluorophotometer (Shimadzu, Kyoto, Japan) with excitation and emission wavelengths 408 and 540 nm (with band widths 5 and 10 nm), as described previously4.
Miscellaneous
Protein concentrations were determined by the method of Lowry et al.37 with bovine serum albumin as a standard. Three-dimensional models of the enzyme were reproduced by the program VMD38. The values presented are the mean ± s.d. (n = 3–4).
Additional Information
How to cite this article: Danko, S. et al. Membrane Perturbation of ADP-insensitive Phosphoenzyme of Ca2+-ATPase Modifies Gathering of Transmembrane Helix M2 with Cytoplasmic Domains and Luminal Gating. Sci. Rep. 7, 41172; doi: 10.1038/srep41172 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Toyoshima, C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 476, 3–11 (2008).
Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 1793, 941–946 (2009).
Møller, J. V., Olesen, C., Winther, A.-M. L. & Nissen, P. The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 (2010).
Danko, S., Yamasaki, K., Daiho, T. & Suzuki, H. Distinct natures of beryllium fluoride-bound, aluminum fluoride-bound, and magnesium fluoride-bound stable analogues of an ADP-insensitive phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 279, 14991–14998 (2004).
Seekoe, T., Peall, S. & McIntosh, D. B. Thapsigargin and dimethyl sulfoxide activate medium Pi - HOH oxygen exchange catalyzed by sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 276, 46737–46744 (2001).
Danko, S., Yamasaki, K., Daiho, T., Suzuki, H. & Toyoshima, C. Organization of cytoplasmic domains of sarcoplasmic reticulum Ca2+-ATPase in E1P and E1ATP states: a limited proteolysis study. FEBS Lett. 505, 129–135 (2001).
Toyoshima, C., Nomura, H. & Tsuda, T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004).
Toyoshima, C., Norimatsu, Y., Iwasawa, S., Tsuda, T. & Ogawa, H. How processing of aspartylphosphate is coupled to lumenal gating of the ion pathway in the calcium pump. Proc. Natl. Acad. Sci. USA 104, 19831–19836 (2007).
Daiho, T., Yamasaki, K., Danko, S. & Suzuki, H. Second transmembrane helix (M2) and long range coupling in Ca2+-ATPase. J. Biol. Chem. 289, 31241–31252 (2014).
Olesen, C., Sørensen, T. L., Nielsen, R. C., Møller, J. V. & Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306, 2251–2255 (2004).
Olesen, C. et al. The structural basis of calcium transport by the calcium pump. Nature 450, 1036–1042 (2007).
Yamasaki, K., Daiho, T., Danko, S. & Suzuki, H. Multiple and distinct effects of mutations of Tyr122, Glu123, Arg324, and Arg334 involved in interactions between the top part of second and fourth transmembrane helices in sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 279, 2202–2210 (2004).
Wang, G., Yamasaki, K., Daiho, T. & Suzuki, H. Critical hydrophobic interactions between phosphorylation and actuator domains of Ca2+-ATPase for hydrolysis of phosphorylated intermediate. J. Biol. Chem. 280, 26508–26516 (2005).
Yamasaki, K., Wang, G., Daiho, T., Danko, S. & Suzuki, H. Roles of Tyr122-hydrophobic cluster and K+ binding in Ca2+-releasing process of ADP-insensitive phosphoenzyme of sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 283, 29144–29155 (2008).
Yamasaki, K., Daiho, T., Danko, S. & Suzuki, H. Assembly of a Tyr122 hydrophobic cluster in sarcoplasmic reticulum Ca2+-ATPase synchronizes Ca2+ affinity reduction and release with phosphoenzyme isomerization. J. Biol. Chem. 290, 27858–27879 (2015).
Danko et al. ADP-insensitive phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase has a compact conformation resistant to proteinase K, V8 protease and trypsin. FEBS Lett. 489, 277–282 (2001).
Danko, S., Daiho, T., Yamasaki, K., Liu, X. & Suzuki, H. Formation of the stable structural analog of ADP-sensitive phosphoenzyme of Ca2+-ATPase with occluded Ca2+ by beryllium fluoride. J. Biol. Chem. 284, 22722–22735 (2009).
Daiho, T., Yamasaki, K., Danko, S. & Suzuki, H. Critical role of Glu40-Ser48 loop linking actuator domain and first transmembrane helix of Ca2+-ATPase in Ca2+ deocclusion and release from ADP-insensitive phosphoenzyme. J. Biol. Chem. 282, 34429–34447 (2007).
Daiho, T., Danko, S., Yamasaki, K. & Suzuki, H. Stable structural analog of Ca2+-ATPase ADP-insensitive phosphoenzyme with occluded Ca2+ formed by elongation of A-domain/M1’-linker and beryllium fluoride binding. J. Biol. Chem. 285, 24538–24547 (2010).
Shigekawa, M. & Pearl, L. J. Activation of calcium transport in skeletal muscle sarcoplasmic reticulum by monovalent cations. J. Biol. Chem. 251, 6947–6952 (1976).
Sorensen, T. L. et al. Localization of a K+ -binding site involved in dephosphorylation of the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 279, 46355–46358 (2004).
Inesi, G., Lewis, D., Toyoshima, C., Hirata, A. & de Meis, L. Conformational fluctuations of the Ca2+-ATPase in the native membrane environment. Effects of pH, temperature, catalytic substrates, and thapsigargin. J. Biol. Chem. 283, 1189–1196 (2008).
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002).
Kato, S. et al. Val200 residue in Lys189–Lys205 outermost loop on the A domain of sarcoplasmic reticulum Ca2+-ATPase is critical for rapid processing of phosphoenzyme intermediate after loss of ADP sensitivity. J. Biol. Chem. 278, 9624–9629 (2003).
Dupont, Y. & Pougeois, R. Evaluation of H2O activity in the free or phosphorylated catalytic site of Ca2+-ATPase. FEBS Lett. 156, 93–98 (1983).
Toyoshima, C., Yonekura, S., Tsueda, J. & Iwasawa, S. Trinitrophenyl derivatives bind differently from parent adenine nucleotides to Ca2+-ATPase in the absence of Ca2+ . Proc. Natl. Acad. Sci. USA. 108, 1833–1838 (2011).
Champeil, P. et al. Kinetic characterization of the normal and detergent-perturbed reaction cycles of the sarcoplasmic reticulum calcium pump. Rate-limiting step(s) under different conditions. J. Biol. Chem. 261, 16372–16384 (1986).
Wakabayashi, S., Ogurusu, T. & Shigekawa, M. Modulation of the hydrolysis rate of the ADP-insensitive phosphoenzyme of the sarcoplasmic reticulum ATPase by H + and Mg2+ . J. Biol. Chem. 262, 9121–9129 (1987).
Bishop, J. E. & Al-Shawi, M. K. Inhibition of sarcoplasmic reticulum Ca2+-ATPase by Mg2+ at high pH. J. Biol. Chem. 263, 1886–1892 (1988).
Toyoshima, C. et al. Crystal structures of the calcium pump and sarcolipin in the Mg2+ -bound E1 state. Nature 495, 260–264 (2013).
Winther, A. M. et al. The sarcolipin-bound calcium pump stabilizes calcium sites exposed to the cytoplasm. Nature 495, 265–269 (2013).
Nakamura, S., Suzuki, H. & Kanazawa, T. The ATP-induced change of tryptophan fluorescence reflects a conformational change upon formation of ADP-sensitive phosphoenzyme in the sarcoplasmic reticulum Ca2+-ATPase. Stopped-flow spectrofluorometry and continuous flow-rapid quenching method. J. Biol. Chem. 269, 16015–16019 (1994).
Barrabin, H., Scofano, H. M. & Inesi, G. Adenosinetriphosphatase site stoichiometry in sarcoplasmic reticulum vesicles and purified enzyme. Biochemistry 23, 1542–1548 (1984).
Weber, K. & Osborn, M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 244, 4406–4412 (1969).
Daiho, T., Suzuki, H., Yamasaki, K., Saino, T. & Kanazawa, T. Mutations of Arg198 in sarcoplasmic reticulum Ca2+-ATPase cause inhibition of hydrolysis of the phosphoenzyme intermediate formed from inorganic phosphate. FEBS Lett. 444, 54–58 (1999).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Juul, B. et al. Do transmembrane segments in proteolyzed sarcoplasmic reticulum Ca2+-ATPase retain their functional Ca2+ binding properties after removal of cytoplasmic fragments by proteinase K? J. Biol. Chem. 270, 20123–20134 (1995).
Brandl, C. J., Green, N. M., Korczak, B. & MacLennan, D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell 44, 597–607 (1986).
Acknowledgements
We thank Dr. David B. McIntosh for improving our manuscript. This work was supported by JSPS KAKENHI Grant Number JP15H04346.
Author information
Authors and Affiliations
Contributions
S.D. and H.S. conceived and coordinated the study and wrote the paper. S.D., K.Y. and H.S. designed, performed and analyzed the experiments. T.D. provided critical discussion. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Danko, S., Yamasaki, K., Daiho, T. et al. Membrane Perturbation of ADP-insensitive Phosphoenzyme of Ca2+-ATPase Modifies Gathering of Transmembrane Helix M2 with Cytoplasmic Domains and Luminal Gating. Sci Rep 7, 41172 (2017). https://doi.org/10.1038/srep41172
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41172
- Springer Nature Limited
This article is cited by
-
Structure and transport mechanism of the human calcium pump SPCA1
Cell Research (2023)
-
Angle change of the A-domain in a single SERCA1a molecule detected by defocused orientation imaging
Scientific Reports (2021)
-
Crystal structures of the gastric proton pump
Nature (2018)