Abstract
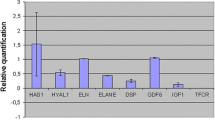
Several reports have shown that a number of cytokines such as tumor necrosis-α (TNF-α), interferon-γ (IFN-γ), and interleukin-β (IL-1β) are capable to induce hyaluronan sinthases (HASs) mRNA expression in different cell culture types. The obvious consequence of this stimulation is a marked increment in hyaluronan (HA) production. It has been also reported that oxidative stress, by itself, may increase HA levels. The aim of this study was to evaluate how TNF-α, IFN-γ,IL−1β, and exposition to oxidative stress may modulate HAS activities in normal human skin fibroblasts. Moreover, the effects on HAS mRNA expression of the concomitant treatment with cytokines and oxidants, and the HA concentrations after treatments, were studied. TNF-α, IFN-γ, and IL-1β were added to normal or/and exposed to FeSO4 plus ascorbate fibroblast cultures and HAS1, HAS2 and HAS3 mRNA content, by PCR-real time, was assayed 3,h later. HA levels were also evaluated after 24,h incubation. The treatment of fibroblasts with cytokines up-regulated HASs gene expression and increased HA production. IL-1β induced HAS mRNA expression and HA production more efficiently than TNF-α and IFN-γ. The exposition of the fibroblasts with the oxidant system markedly increased HAS activities while slightly HA production. The concomitant treatment of cells with the cytokines and the oxidant was able to further enhance, in a dose dependent way, with synergistic effect on HAS mRNA expression. On the contrary HA levels resulted unaffected by the concomitant treatment, and resemble those obtained with the exposure to FeSO4 plus ascorbate only. This lack in HA production could be due to the deleterious action of free radicals on the HA synthesis.
Similar content being viewed by others
Abbreviations
- GAGs:
-
glycosaminoglycans
- PGs:
-
proteoglycans
- ECM:
-
extracellular matrix
- HA:
-
hyaluronic acid
- HASs:
-
hyaluronan synthases
- TNF-α:
-
tumor necrosis factor alpha
- IFN-γ:
-
interferon gamma
- IL-1β:
-
interleukin beta
- ROS:
-
reactive oxygen species
- DMEM:
-
Dulbecco’s minimal essential medium
- FBS:
-
foetal bovine serum
- NADH:
-
reduced nicotinamide adenine dinucleotide
- EDTA:
-
ethylenediaminetetraacetic acid
- SDS:
-
sodium dodecylsulphate
- PBS:
-
buffer phosphate saline
- S.D.:
-
standard deviation
References
Iozzo RV: Matrix proteoglycans: From molecular design to cellular function. Annu Rev Biochem 67: 609–652, 1998
Knudson CB, Knudson W: Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J 7: 1233–1241, 1993
Weigel PH, Hascall VC, Tammi M: Hyaluronan Synthase. J Biol Chem 272: 13997–14000, 1997
Itano N, Sawait T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spider PA, McDonald AJ, Kimata K: Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274: 25085–25092, 1999
Oguchi T, Ishiguro N: Differential stimulation of three forms of hyaluronan synthase by TGF-β, IL-1β, and TNF-α. Connect Tissue Res 45: 197–205, 2004
Yamada Y, Itano N, Hata K, Ueda M, Kimata K: Differential regulation by IL-1β and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol 122: 631–639, 2004
Stuhlmeier KM, Pollaschek C: Differential effect of transforming growth factor beta (TGF-β) on the genes encodinghyaluronan synthases and utilization of the p38 MAPK pathway in TGF-β-induced hyaluronan synthase 1 activation. J BiolChem 279: 8753–8760, 2004
Anastassiades TP, Wood A: Effect of soluble products from lectin stimulated lymphocytes on thegrowth adhesiveness and glycosaminoglycan synthesis of cultured synovial fibroblastic cells. JClin Invest 68: 792–802, 1981
Whiteside TL, Worrall JG, Prince RK, Buckingham RB, Rodman GP: Soluble mediators from mononuclear cells increase the synthesis of glycosaminoglycans by dermal fobroblasts from normal subjects and progressive systemic sclerosis patients. Arthritis Rheum 28: 188–197, 1985
Droge W: Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002
Collis CS, Yang M, Peach SJ, Diplock AT, Rice-Evans C: The effects of ascorbic acid and iron co-supplementation on the proliferation of 3T3 fibroblasts. Free Radic Res 25: 87–93, 1996
Bustin SA: Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25: 169–193, 2000
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN: Intracellular hyaluronan: a newfrontier for inflammation? Biochim Biophys Acta 1763: 3–12, 2004
Laurent TC, Fraser JRE: Catabolism of hyaluronan. In: Degradation of bioactive substances:physiology and pathology. In: JHHenriksen (ed.). CRC Press, Boca Raton, pp. 249–265, 1991
Spicer AP, Nguyen TK: Mammalian hyaluronan synthases: investigation of functional relationships in vivo. Biochem Soc Trans 27: 109–115, 1999
Laurent TC, Fraser JRE: Hyaluronan. FASEB J 6: 2397–2404, 1992
Iozzo RV, Murdoch AD: Proteoglycans of the extracellular environment: clues from the gene and protein side offer novelperspectives in molecular diversity and function. FASEB J 10: 598–614, 1996
Campo GM, Avenoso A, D'Ascola A, Campo S, Ferlazzo AM, Samà D, Calatroni A: Purified human plasma glycosaminoglycans limit oxidative injury induced by iron plus ascorbate in skin fibroblast cultures. Toxicol In Vitro 19: 561–572, 2005
Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A: Efficacy of treatment withglycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther 5:R122–R131, 2003
Suzuki Y, Yamaguchi T: Effects of hyaluronic acid on macrophage phagocytosis and active oxygenrelease. Agents Actions 38: 32–37, 1993
Kvam BJ, Fragonas E, Degrassi A, Kvam C, Matulova M, Pollesello P, Zanetti F, Vittur F: Oxygen-derived free radical (ODFR)action on hyaluronan (HA), on two HA ester derivatives, and on the metabolism of articular chondrocytes. Exp Cell Res 218:79–86, 1995
Fukuda K, Takayama M, Ueno M, Oh M, Asada S, Kumano F, Tanaka S: Hyaluronic acid inhibits interleukin-1-induced superoxide anion in bovine chondrocytes. Inflamm Res 46: 114–117, 1997
Calatroni A: Extraction and purification of glycosaminoglycans (GAGs) from biological fluids. In: Analytical techniques toevaluate the structure and function of natural polysaccharides, glycosaminoglycans. In: N Volpi (ed.). Research SignpostPress, Trivandrum., pp. 15–22, 2002
Friman C, Nordstrom D, Eronen I: Plasma glycosaminoglycans in systemic lupus erythematosus. J Rheumatol 14: 1132–1134, 1987
Laurent TC, Laurent UBG, Fraser JRE: Serum hyaluronan as a disease marker. Ann Med 28: 241–253, 1996
Radhakrishnamurthy B, Tracy RE, Dalferes Jr, Berenson GS: Proteoglycans in human coronary arteriosclerotic lesions. Exp Mol Pathol 65: 1–8, 1998
Calabrò L, Musolino C, Spatari G, Vinci R, Calatroni A: Increased concentration of circulating acid glycosaminoglycans in chronic lymphocytic leukaemia and essential thrombocythaemia. Clin Chim Acta 269: 185–99, 1998
Roughley PJ: Articular cartilage and changes in arthritis: noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res 3: 342–347, 2001
Plevris JN, Haydon GH, Simpson KJ, Dawkes R, Ludlum CA, Harrison DJ, Hayes PC: Serum hyaluronan: a non invasive test fordiagnosing liver cirrhosis. Eur J Gastroenterol Hepatol 12: 1121–1127, 2000
Elias JA, Jimenez SA, Freundlich B: Recombinant gamma, alpha and beta interferon regulation ofhuman lung fibroblast growth. Am Rev Respir Dis 135: 62–65, 1987
Palladino Jr, Shepard HM: Recombinant human tumor necrosis factor-alpha. Effects on proliferationof normal and transformed cells in vitro. Science 230: 943–945, 1985
Libby P, Friedman GB, Salomon RN: Cytokines as modulators of cell proliferation in fibroticdiseases. Am Rev Respir Dis 140: 1114–1117, 1989
Mantovani A, Bussolino F, Introna M: Cytokine regulation of endothelial cell function: frommolecular level to the bedside. Immunol Today 18: 231–240, 1997
Tanimoto K, Ohno S, Fujimoto K, Honda K, Ijuin C, Tanaka N, Doi T, Nakahara M, Tanne K: Proinflammatory cytokines regulate thegene expression of hyaluronic acid synthases in cultured rabbit synovial membrane cells. Connect Tissue Res 42: 187–195, 2001
Wilkinson TS, Potter-Perigo S, Tsoi C, Altman CL, Wight TN: Pro- and anti-inflammatory factorscooperate to control hyaluronan synthesis in lung fibroblasts. Am J Resp Cell Mol Biol 31: 92–99, 2004
Jacobson A, Brinck J, Brinskin MJ, Spicer AP, Heldin P: Expression of human hyaluronan synthases in response to external stimuli. Biochem J 348: 29–35, 2000
Ducale AE, Ward SI, Dechert T, Yager DR: Regulation of hyaluronan synthase-2 expression in human intestinal mesenchymal cells:mechanisms of interleukin-1β-mediated induction. Am J Physiol Gastrointest Liver Physiol 289: G462-G470, 2005
Remick DG, Villarete L: Regulation of cytokine gene expression by reactive oxygen and reactivenitrogen intermediates. J Leukoc Biol 59: 471–475, 1996
Haralson MA, Hassell JR: The extracellular matrix: an overview. In: Extracellular matrix — a pratical approach. In: MAHaralson, JR Hassell (eds.). IRL Press, New York, pp. 1–30, 1995
Atamas SP: Complex cytokine regulation of tissue fibrosis. Life Sci 72: 631–643, 2002
Bendall F: Chemokines and their receptor in diseases. Histol Histopathol 20: 907–926, 2005
Moulton PJ: Inflammatory joint disease: the role of cytokines, cyclooxygenases and reactive oxygen species. Br J Biomed Sci53: 317–324, 1996
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campo, G.M., Avenoso, A., Campo, S. et al. TNF-α, IFN-γ, and IL−1β modulate hyaluronan synthase expression in human skin fibroblasts: Synergistic effect by concomital treatment with FeSO4 plus ascorbate. Mol Cell Biochem 292, 169–178 (2006). https://doi.org/10.1007/s11010-006-9230-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9230-7