Abstract
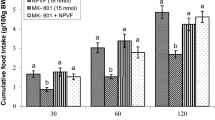
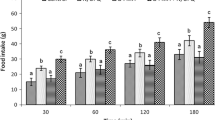
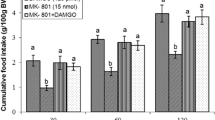
This study was designed to determine possible interaction of the central nociceptin/orphanin FQ (N/OFQ) and glutamatergic system on feeding behavior in neonatal broilers chicken. In experiment 1, 3-h food deprived (FD3) chicks received intracerebroventricular (ICV) injection of (i) control solution, (ii) N/OFQ (16 nmol), (iii) MK-801 (NMDA [N-methyl-d-aspartate-type glutamate] receptor antagonist, 15 nmol) and (iv) N/OFQ + MK-801. Experiments 2–7 were similar to experiment 1, except chicken ICV injected with CNQX (AMPA [α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid] receptor antagonist, 390 nmol), UBP-302 (kainate receptor antagonist, 390 nmol), AIDA (mGluR1 antagonist, 2 nmol), LY341495 (mGluR2 antagonist, 150 nmol), UBP1112 (mGluR3 antagonist, 2 nmol) and Glutamate (300 nmol) instead of MK-801. Then the cumulative food intake measured until 120 min post injection. According to the results, ICV injection of N/OFQ significantly increased food intake (P < 0.05). Co-administration of the N/OFQ + MK-801 significantly amplified N/OFQ-induced hyperphagia in neonatal broilers (P < 0.05). Injection of the N/OFQ + CNQX significantly increased hyperphagic effect of the CNQX in neonatal meat-type chicken (P < 0.05). Co-injection of the N/OFQ + glutamate significantly decreased N/OFQ-induced hyperphagia in neonatal meat type chicken (P < 0.05). These results suggested interconnection between central N/OFQ and glutamatergic systems on feeding behavior mediates via NMDA and AMPA receptors in neonatal broilers.









Similar content being viewed by others
References
Abbasnejad M, Jonaidi H, Pourrahimi AM (2005) Feeding and locomotion responses to centrally injected nociceptine/orphanin FQ in chicks. Physiol Behav 85:383–386
Alt C, Lam JS, Harrison MT, Kershaw KM, Samuelsson S, Toll L, D’Andrea A (2012) Nociceptin/orphanin FQ inhibition with SB612111 ameliorates dextran sodium sulfate-induced colitis. Eur J Pharmacol 683:285–293
Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M et al (2013) Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med 5:173–188
Baghbanzadeh A, Babapour V (2007) Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. J Vet Res 62(4):125–129
Bayrakdar ET, Bojnik E, Armagan G, Kanit L, Benyhe S, Borsodi A, Yalcin A (2013) Kainic acid-induced seizure activity alters the mRNA expression and G-protein activation of the opioid/nociceptin receptors in the rat brain cortex. Epilepsy Res 105:13–19
Blevins JE, Stanley BG, Reidelberger RD (2002) DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 71:277–282
Bregola G, Candeletti S, Romualdi P, Simonato M (1999) Limbic seizures increase pronociceptin mRNA levels in the thalamic reticular nucleus. Neuroreport 10:541–546
Bungo T, Shiraishi J, Yanagita K, Ohta Y, Fujita M (2009) Effect of Nociceptin/Orphanin FQ on feeding behavior and hypothalamic neuropeptide expression in layer-type chicks. Gen Comp Endocrinol 163:47–51
Charles JR, Duva MA, Ramirez GJ, Lara RL, Yang CR, Stanley BG (2014) Activation of lateral hypothalamic mGlu1 and mGlu5 receptors elicits feeding in rats. Neuropharmacology 79:59–65
Da Silva AA, Marino-Neto J, MA P (2003) Feeding induced by microinjections of NMDA and AMPA–kainite receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain Res 966:76–83
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Farhang B, Pietruszewski L, Lutfy K, Wagner EJ (2010) The role of the nop receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology 59(3):190–200
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Goeldner C, Reiss D, Wichmann J, Meziane H, Kieffer BL, Ouagazzal AM (2008) Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. J Neurosci 28:2190–2198
Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM (2009) Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol Learn Mem 91(4):393–401
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56(4):443–451
Hettes SR, Gonzaga WJ, Heyming TW, Nguyen JK, Perez S, Stanley BG (2010) Stimulation of lateral hypothalamic AMPA receptors may induce feeding in rats. Brain Res 1346:112–120
Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M (2014) Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology 39:1081–1092
Levine AS (2006) The animal model in food intake regulation: examples from the opioid literature. Physiol Behav 89:92–96
Marti M, Guerrini R, Beani L, Bianchi C, Morari M (2002) Nociceptin/orphanin FQ receptors modulate glutamate extracellular levels in the substantia nigra pars reticulata. a microdialysis study in the awake freely moving rat. Neuroscience 112(1):153–160
McFadden KL, Cornier MA, Tregellas JR (2014) The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol 5(553):1–7
Meyer LC, Paisley CE, Mohamed E, Bigbee JW, Kordula T, Richard H, Lutfy K, Sato-Bigbee C (2017) Novel role of the nociceptin system as a regulator of glutamate transporter expression in developing astrocytes. Glia 65:2003–2023
Nicholson JR, Akil H, Watson SJ (2002) Orphanin FQ-induced hyperphagia is mediated by corticosterone and central glucocorticoid receptors. Neuroscience 115:637–643
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J Poult Sci 5(4):301–308
Olszewski PK, Grace MK, Sanders JB, Billington CJ, Levine AS (2002) Effect of Nociceptin/orphanin FQ on food intake in rats that differ in diet preference. Pharmacol Biochem Behav 73(3):529–535
Olszewski PK, Grace MK, Shirazi Fard S, Greve`s ML, Klockars A, Massi M, Schiöth HB, Levine AS (2010) Central nociceptin/orphanin FQ system elevates food consumption by both increasing energy intake and reducing aversive responsiveness. Am J Physiol Regul Integr Comp Physiol 299:R655–R663
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intraaccumbens opioid administration. Behav Brain Res 260:131–138
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hearing Res 236:52–60
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270(5237):792–794
Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK (2007) Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learn Mem 14:29–35
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Seyedali Mortezaei S, Zendehdel M, Babapour V, Hasani K (2013) The role of glutamatergic and GABAergic systems on serotonin-induced feeding behavior in chicken. Vet Res Commun 37:303–310
Stratford TR, Holahan MR, Kelley AE (1997) Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. Neuroreport 8(2):423–426
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DLAP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67:217–223
Tajalli S, Jonaidi H, Abbasnejad M, Denbow DM (2006) Interaction between nociceptin/orphanin FQ (N/OFQ) and GABA in response to feeding. Physiol Behav 89:410–413
Tallent MK (2008) Presynaptic inhibition of glutamate release by neuropeptides: use-dependent synaptic modification. Results Probl Cell Differ 44:177–200
Toll L, Bruchas MR, Calo’ G, Cox BM, Zaveri NT (2016) Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 68:419–457
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Werthwein S, Bauer U, Nakazi M, Kathmann M, Schlicker E (1999) Further characterization of the ORL1 receptor-mediated inhibition of noradrenaline release in the mouse brain in vitro. Br J Pharmacol 127:300–308
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behaviour in broiler cockerels. J Comp Physiol A 195:715–720
Zendehdel M, Hamidi F, Hassanpour S (2013a) The effect of histaminergic system on Nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther 21:179–186
Zendehdel M, Mokhtarpouriani K, Babapour V, Baghbanzadeh A, Pourrahimi M, Hassanpour S (2013b) The effect of serotonergic system on nociceptin/orphanin FQ induced food intake in chicken. J Physiol Sci 63:271–277
Acknowledgements
The authors thank the central laboratory (Dr. Rastegar Lab.) of the Faculty of Veterinary Medicine, University of Tehran for cooperation. This research is conducted as a part of the PhD thesis of the first author.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and Animal Rights
All experiments were executed according to the Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Abolghasempour, S., Zendehdel, M., Panahi, N. et al. Intracerebroventricular Injection of the Glutamatergic Receptors Antagonist Affects N/OFQ-Induced Hyperphagia in Neonatal Broilers: Role of NMDA and AMPA Receptors. Int J Pept Res Ther 25, 835–843 (2019). https://doi.org/10.1007/s10989-018-9733-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-018-9733-6