Abstract
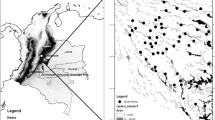
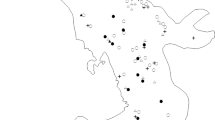
The understanding and prediction of the responses of animal populations to habitat fragmentation is a central issue in applied ecology. The identification of habitat variables associated to patch occupancy is particularly important when habitat quality is affected by human activities. Here, we analyze the influence of patch and landscape characteristics on patch occupancy by the subterranean herbivorous rodent Ctenomys porteousi. Patch occupancy was monitored in a network of 63 habitat patches identified by satellite imagery analysis which extends along almost the whole distributional range for C. porteousi. Suitable habitat for the occurrence of C. porteousi is highly fragmented and represents <10% of the total area in its distributional range. The distribution of C. porteousi in the patch network is affected not only by characteristics of the habitat patches, but also by those of the surrounding landscape matrix. Significant differences between occupied and empty patches were found in several environmental variables. Overall, occupied patches were larger, less vegetated, more connected, and had larger neighbor patches than empty patches. A stepwise procedure on a generalized linear model selected four habitat variables that explain patch occupancy in C. porteousi; it included the effects of habitat quality in the matrix surrounding the patch, average vegetation cover in the patch, minimum vegetation cover in the matrix surrounding the patch, and the area of the nearest neighbor patch. These results indicate that patch occupancy in C. porteousi is strongly influenced by the availability and quality of habitat both in the patch and in the surrounding landscape matrix.

Similar content being viewed by others
References
Andren H, Delin A, Seiler A (1997) Population response to landscape changes depends on specialization to different landscape elements. Oikos 80:193–196
Antinuchi CD, Busch C (1992) Burrow structure in the subterranean rodent Ctenomys talarum. Z Säugetirkd 57:163–168
Antinuchi CD, Zenuto RR, Luna F, Cutrera AP, Perisinotti P, Busch C (2006) Energy budget in subterranean rodents: insights from the tuco-tuco Ctenomys talarum (Rodentia: Ctenomyidae). In Kelt DA, Salazar-Bravo JA, Patton JL (eds) The quintessential naturalist: honoring the life and legacy of Oliver Pearson. University of California Press, Berkeley, CA, pp 111–140
Anton DJ (1995) Diversity, globalization, and the ways of nature. International Development Research Centre, Canada
Anzures-Dadda A, Manson RH (2007) Patch- and landscape-scale effects on howler monkey distribution and abundance in rainforest fragments. Anim Conserv 10:69–76
Arnold GW, Weeldenburg JR, Ng VM (1995) Factors affecting the distribution and abundance of Western grey kangaroos (Macropus fuliginosus) and euros (M. robustus) in a fragmented landscape. Landscape Ecol 10:65–74
Bergl RA, Vigilant L (2007) Genetic analysis reveals population structure and recent migration within the highly fragmented range of the Cross River gorilla (Gorilla gorilla diehli). Mol Ecol 16:501–516
Bivand R (2009) The Spdep package. Comprehensive R Archive Network, Version 0.4-34
Burnham KP, Anderson DR (2002) Model Selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York
Busch C, Antinuchi CD, del Valle JC, Kittlein MJ, Malizia AI, Vassallo AI, Zenuto RR (2000) Population ecology of subterranean rodents. In Lacey EA, Patton JL, Cameron GN (eds) Life underground: the biology of subterranean rodents. University of Chicago Press, pp 183–226
Clark-Laboratories (1999). Idrisi32. Clark University, Worcester, MA
Comparatore VM, Cid MS, Busch C (1995) Dietary preferences of two sympatric subterranean rodent populations in Argentina. Rev Chil Hist Nat 68:197–206
Cutrera AP, Antinuchi CD, Mora MS, Vassallo AI (2006) Home-range and activity patterns of the South American subterranean rodent Ctenomys talarum. J Mamm 87:1183–1191
Davies KF, Melbouurne BA (2007) The tails of two geckos tell the sstory of dispersal in fragmented landscape. Mol Ecol 16:3289–3291
Delin AE, Andrén H (1999) Effects of habitat fragmentation on Eurasian red squirrel (Sciurus vulgaris) in a forest landscape. Landscape Ecol 14:67–72
Díaz GB, Ojeda RA (2000) Libro rojo de mam’ıferos amenazados de la Argentina. Sociedad Argentina para el Estudio de los Mam’ıferos, Mendoza, Argentina
Fernández-Stolz GP, Stolz JFB, Freitas TRO (2007) Bottlenecks and dispersal in the tuco-tuco das dunas, Ctenomys flamarioni (Rodentia: Ctenomyidae), in Southern Brazil. J Mamm 88:935–945
Fleishman E, Ray C, Sjogren-Gulve P, Boggs CL, Murphy DD (2002) Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv Biol 16:706–716
Franken, RJ, Hik DS (2004) Influence of habitat quality, patch size and connectivity on colonization and extinction dynamics of collared pikas Ochotona collaris. J Anim Ecol 73:889–896
Guadagnin DL, Maltchik L (2007) Habitat and landscape factors associated with neotropical waterbird occurrence and richness in wetland fragments. Biodivers Conserv 16:1231–1244
Haining RP (2003) Spatial data analysis: theory and practice. Cambridge University Press, Cambridge, MA
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63:151–162
Hanski I (1999) Metapopulation ecology. Oxford University Press, New York
Hanski I, Kuussaari M, Nieminen M (1994) Metapopulation structure and migration in the butterfly Melitaea cinxia. Ecology 75:747–762
Hanski I, Pakkala T, Kuussaari M, Guangchun, L (1995) Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 72:21–28
Henein K, Merriam G (1990) The elements of connectivity where corridor quality is variable. Landscape Ecol 4:157–170
Hokit DG, Stith BM, Branch LC (1999) Effects of landscape structure in Florida scrub: a population perspective. Ecol Appl 9:124–134
Hokit DG, Stith BM, Branch LC (2001) Comparison of two types of metapopulation models in real and artificial landscapes. Conserv Biol 15:1102–1113
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Ims R (1995) Movement patterns related to spatial structures. In: Hansson L, Fahrig L, Merriam G (eds) Mosaic landscapes and ecological processes. Chapman and Hall, London, pp 85–109
Jonsen I, Bourchier R, Roland J (2001) The influence of matrix habitat on Aphthona flea beetle immigration to leafy spurge patches. Oecologia 127:287–294
Kittlein MJ, Gaggiotti OE (2008) Interactions between environmental factors can hide isolation by distance patterns: a case study of Ctenomys rionegrensis in Uruguay. Proc R Soc Lond B 75:2633–2638
Lacey EA (2000) Spatial and social systems of subterranean rodents. In: Lacey EA, Patton JL, Cameron GN (eds) Life underground: the biology of subterranean rodents. University of Chicago Press, Chicago, pp 257–296
Laurance WF, Bierregaard RO (1997) Tropical forest remnants: ecology, management, and conservation of fragmented communities. University of Chicago Press, Chicago
Mac Nally R, Bennett AF, Horrocks G (2000) Forecasting the impacts of habitat fragmentation. Evaluation of species-specific predictions of the impact of habitat fragmentation on birds in the box–ironbark forests of central Victoria, Australia. Biol Conserv 95:7–29
Mazerolle MJ, Villard M (1999) Patch characteristics and landscape context as predictors of species presence and abundance: a review. Ecoscience 6:117–124
McCullagh P, Nelder J (1983) Generalized linear models. Chapman and Hall, Cambridge
Merriam G, Kozakiewicz M, Tsuchiya E, Hawley K (1989) Barriers as boundaries for metapopulations and demes of Peromyscus leucopus in farm landscapes. Landscape Ecol 2:227–235
Moilanen A, and Hanski I (2001) On the use of connectivity measures in spatial ecology. Oikos 95:147–151
Mora MS (2008) Biología Metapoblacional del tuco-tuco de las dunas (Ctenomys australis): Efectos de la estructura espacial del hábitat sobre la ecología y genética poblacional. PhD thesis, Universidad Nacional de Mar del Plata, Argentina
Mora MS, Lessa EP, Cutrera AP, Kittlein MJ, Vassallo AI (2007) Phylogeographic structure in the subterranean tuco-tuco Ctenomys talarum (Rodentia: Ctenomyidae): contrasting the demographic consequences of regional and habitat-specific histories. Mol Ecol 16:3453–3465
Opdam P, van Apeldoorn R, Schotman A, Kalkhoven J (1993) Population responses to landscape fragmentation. In: Vos CC, Opdam P (ed) Landscape ecology of a stressed environment. Chapman and Hall, London, pp 147–167
Patterson BD, Ceballos G, Sechrest W, Tognelli MF, Brooks T, Luna L, Ortega P, Salazar I, Young BE (2003) Digital distribution maps of the mammals of the Western Hemisphere, version 1.0. NatureServe, Arlington, Virginia
Peltonen A, Hanski I (1991) Patterns of island occupancy explained by colonization and extinction rates in shrews. Ecology 72:1698–1708
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190(3–4):231–259
Reunanen P, Nikula A, Mönkkönen M, Hurme E, Nivala V (2002) Predicting occupancy for the siberian flying squirrel in old-growth forest patches. Ecol Appl 12:1188–1198
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Rouse JW, Haas RH, Schell JA, Deering DW, Harlan JC (1974) Monitoring the vernal advancement of retrogradation of natural vegetation. Technical report, NASA/GSFC, Type III, Final Report, Greenbelt, MD, USA
Sjögren-Gulve P, Ray C (1996) Using logistic regression to model metapopulation dynamics: large-scale forestry extirpates the pool frog. In: McCullough DR (ed) Metapopulations and wildlife conservation. Island Press, Washington, pp 111–137
Stolz JF (2006) Dinâmica populacional e relações espaciais do tuco-tuco-das-dunas (Ctenomys flamarioni—Rodentia— Ctenomyidae) na estação ecológica do Taim-RS/Brasil. Master’s thesis, Universidade Federal do Rio Grande do Sul, Brasil
Sutherland GD, Harestad AS, Price K, Lertzman KP (2000) Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv Ecol 4:16–21
Taylor PD, Fahrig L, Henein K, Merriam, G (1993) Connectivity is a vital element of landscape structure. Oikos 68:571–573
van Apeldoorn RC, Celada C, Nieuwenhuizen W (1994) Distribution and dynamics of the red squirrel (Sciurus vulgaris L.) in a landscape with fragmented habitat. Landscape Ecol 9:227–235
Verbeylen G, De Bruyn L, Maticen E (2003) Patch occupancy, population density and dynamics in a fragmented red squirrel Sciurus vulgaris population. Ecography 26:118–128
Verboom J, Schotman A, Opdam P, Metz JAJ (1991) European nuthatch metapopulations in a fragmented agricultural landscape. Oikos 61:149–156
Vleck D (1979) The energy cost of burrowing by the pocket gopher Thomomys bottae. Physiol Zool 52:122–134
Vleck D (1981) Burrow structure and foraging cost in the fossorial rodent, Thomomys bottae. Oecologia 49:391–396
Vos CC, Stumpel AHP (1996) Comparison of habitat-isolation parameters in relation to fragmented distribution patterns in the tree frog (Hyla arborea). Landscape Ecol 11:203–214
Walker RS, Novaro AJ, Branch LC (2003) Effects of patch attributes, barriers, and distance between patches on the distribution of a rock-dwelling rodent (Lagidium viscacia). Landscape Ecol 18:185–192
Watling JI, Donnelly MA (2006) Fragments as islands: a synthesis of faunal responses to habitat patchiness. Conserv Biol 20:1016–1025
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3:385–397
Wiens JA (1997) Metapopulation dynamics and landscape ecology. In: Hanski I, Gilpin ME (eds) Metapopulation biology: ecology, genetics and evolution. Academic Press, Toronto, pp 43–62
Wiens JA, Stenseth NC, Van Horne B, Ims RA (1993) Ecological mechanisms and landscape ecology. Oikos 66:369–380
Wlasiuk G, Garza JC, Lessa EP (2003) Genetic and geographic differentiation in the Rio Negro tuco-tuco (Ctenomys rionegrensis): inferring the roles of migration and drift from multiple genetic markers. Evolution 57:913–926
Acknowledgments
This research was supported by grants from FONCYT (PICT 05-38361) and CONICET (PIP 5844) Argentina. The authors would like to express our gratitude to Matías S. Mora for assistance during fieldwork. FJM is a fellow and MJK is adjunct researcher from CONICET, Argentina. The author thank G. P. Fernández-Stolz and anonymous reviewer for their comments which greatly improved the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mapelli, F.J., Kittlein, M.J. Influence of patch and landscape characteristics on the distribution of the subterranean rodent Ctenomys porteousi . Landscape Ecol 24, 723–733 (2009). https://doi.org/10.1007/s10980-009-9352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-009-9352-x